11734334001
Roche
Universal Protease Substrate
lyophilized, suitable for detection, pkg of 40 mg (40 μmol; 100mM)
Synonym(s):
Substrate of protease, casein, resorufin-labeled, protease substrate, universal
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
UNSPSC Code:
12352204
Recommended Products
form
lyophilized
Quality Level
packaging
pkg of 40 mg (40 μmol; 100mM)
manufacturer/tradename
Roche
concentration
1 mg/mL
application(s)
detection
shipped in
dry ice
storage temp.
−20°C
Related Categories
General description
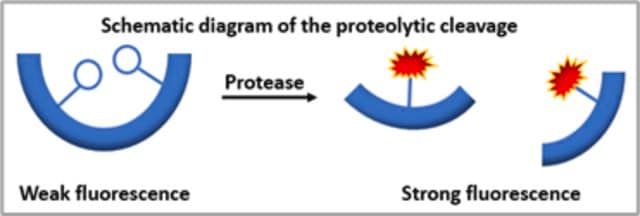
The Universal Protease Substrate provides rapid and highly sensitive detection of trace protease activity, or determination of the effectiveness of protease inhibition. Protease activity releases resorufin-labeled peptides from the patented substrate, resorufin-labeled casein. Resorufin is N-(resorufin-4-carbonyl)-piperidine-4-carbonic acid).The concentration of these labeled peptides in the supernatant is directly related to the proteolytic activity present.
- Conveniently detect nanogram quantities of proteolytic activity in less than one hour.
- Perform highly sensitive protease detection in a homogeneous assay.
Application
Principle
By treatment with proteases, resorufin-labeled peptides are released from resorufin-labeled casein. They cannot be precipitated by trichloroacetic acid. The concentration of these resorufin-labeled peptides in the supernatant is equivalent to the proteolytic activity present.
By treatment with proteases, resorufin-labeled peptides are released from resorufin-labeled casein. They cannot be precipitated by trichloroacetic acid. The concentration of these resorufin-labeled peptides in the supernatant is equivalent to the proteolytic activity present.
Use Universal Protease Substrate as a general substrate for proteases, and to detect trace protease activities, for example, as a contamination in enzyme preparations. It can be measured spectrophotometrically and fluorimetrically in a homogeneous assay.
Quality
Function test: performance-controlled in the assay for proteases.
Preparation Note
Working concentration: Approximately 1 mg/ml
Storage conditions (working solution): -15 to -25 °C
An aqueous solution is stable for several months at -15 to -25° C and for 2 to 3 days at 2 to 8° C. It is recommended to store aqueous solutions in aliquots at -15 to -25° C. Repeated freezing and thawing is possible. At 15 to 25 °C the product is rapidly hydrolyzed in solution.
Storage conditions (working solution): -15 to -25 °C
An aqueous solution is stable for several months at -15 to -25° C and for 2 to 3 days at 2 to 8° C. It is recommended to store aqueous solutions in aliquots at -15 to -25° C. Repeated freezing and thawing is possible. At 15 to 25 °C the product is rapidly hydrolyzed in solution.
Other Notes
For life science research only. Not for use in diagnostic procedures.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Airborne Fungi Induce Nasal Polyp Epithelial Cell Activation and Toll-Like Receptor Expression
Shin, et al.
International Archives of Allergy and Immunology, 153 (1), 46-52 (2010)
Differential Proinflammatory Responses to Aspergillus fumigatus by Airway Epithelial Cells In Vitro Are Protease Dependent
Rowley, et al.
Journal of Fungi, 7, 468-468 (2021)
Cryo-EM structure of the entire FtsH-HflKC AAA protease complex
Qiao, et al.
Cell Reports, 39 (2022)
Anna V Luebben et al.
Science advances, 8(15), eabj8633-eabj8633 (2022-04-16)
Genetic CLN5 variants are associated with childhood neurodegeneration and Alzheimer's disease; however, the molecular function of ceroid lipofuscinosis neuronal protein 5 (Cln5) is unknown. We solved the Cln5 crystal structure and identified a region homologous to the catalytic domain of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service