810600P
Avanti
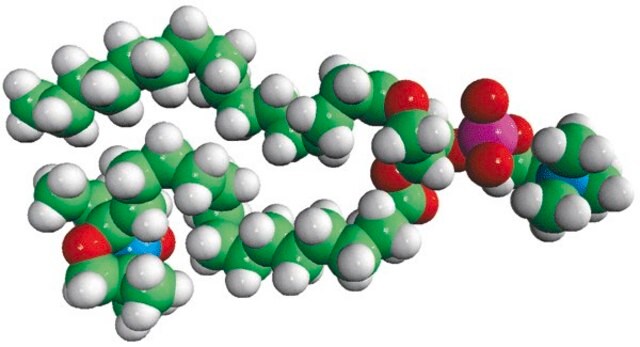
16:0-12 Doxyl PC
Avanti Research™ - A Croda Brand 810600P, powder
Synonym(s):
1-palmitoyl-2-stearoyl-(12-doxyl)-sn-glycero-3-phosphocholine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C46H90N2O10P
CAS Number:
Molecular Weight:
862.19
UNSPSC Code:
41141825
NACRES:
NA.25
Recommended Products
Assay
>99% (TLC)
form
powder
packaging
pkg of 1 × 1 mg (810600P-1mg)
manufacturer/tradename
Avanti Research™ - A Croda Brand 810600P
lipid type
ESR probes
phospholipids
shipped in
dry ice
storage temp.
−20°C
General description
Avanti′s nitroxide spin product listing is a group of compounds designed to act as membrane probes. A variety of positions down the hydrophobic chain are labeled with the nitroxide functional groups to allow probing the membrane at various depths. These compounds have been synthesized from 1-palmitoyl-2-hydroxy-sn-glycerol-3-phosphocholine with the product being purified by column chromatography. Various n-doxyl phosphocholines have been recently used as biophysical tools to elucidate membrane trafficking with phosphatidylinositol transfer proteins and as fluorescent quenchers in lipid bilayer structural studies.
Phosphatidylcholine (PC) is a strong bilayer-forming lipid. It the most common phospholipid in mammalian membranes. It is also an important component of the mucosal layer of the colon. 1-palmitoyl-2-stearoyl-(12-doxyl)-sn-glycero-3-phosphocholine (12-NO-PC) is a nitroxide-labeled phospholipid.
Application
16:0-12 Doxyl PC is suitable for use:
- as a lipophilic collisional quencher to prepare liposomes used in lipophilic quenching experiments
- to prepare liposomes used in nitroxide quenching experiments to examine the location of each NBD (7-nitrobenz-2-oxa-1,3-diazole) probe in mutants
- as a component in POPC or 1:1 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC)/1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-rac-1-glycerol (POPG) mixtures to prepare unlabelled large unilamellar liposomes
Biochem/physiol Actions
Phosphatidylcholine (PC) functions as a surfactant within the mucus to form a hydrophobic surface to inhibit bacterial penetrance. It is used to treat fat embolism. Phosphatidylcholine lowers the levels of cholesterol and triglycerides.
Packaging
5 mL Clear Glass Sealed Ampule (810600P-1mg)
Preparation Note
To prevent aggregation, prepare water-based solutions of 2 mM stock solutions of n-DOXYL PCs and store in plastic. Dilute stock solutions to 0.03- 0.1 mM solutions for EPR studies. For liposome preparations in fluorescent quenching measurements, dissolve the doxyl lipid in 150 μl absolute ethanol for a concentration of 40.3 mM. href="https://pubs.acs.org/doi/suppl/10.1021/ja804929m/suppl_file/ja804929m_si_001.pdf"target="_blank">Supplemental information
Legal Information
Avanti Research is a trademark of Avanti Polar Lipids, LLC
also commonly purchased with this product
Product No.
Description
Pricing
Storage Class Code
11 - Combustible Solids
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Structural insights into the membrane-anchoring mechanism of a cholesterol-dependent cytolysin
Ramachandran R, et al.
Nature Structural and Molecular Biology, 9(11), 823-823 (2002)
The Membranes of Cells, 154(2), 120-128 (2016)
Integrative Medicine - E-Book, 154(2), 120-128 (2017)
Lipid-specific binding of the calcium-dependent antibiotic daptomycin leads to changes in lipid polymorphism of model membranes
Jung D, et al.
Chemistry and Physics of Lipids, 154(2), 120-128 (2008)
The mechanism of membrane insertion for a cholesterol-dependent cytolysin: a novel paradigm for pore-forming toxins
Shatursky O, et al.
Cell, 99(3), 293-299 (1999)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service