W272205
Methyl myristate
≥98%, FG
Synonym(s):
Methyl tetradecanoate, Myristic acid methyl ester
About This Item
Recommended Products
biological source
synthetic
Quality Level
grade
FG
Kosher
Agency
meets purity specifications of JECFA
reg. compliance
EU Regulation 1334/2008 & 178/2002
FDA 21 CFR 172.515
Assay
≥98%
refractive index
n20/D 1.436 (lit.)
bp
323 °C (lit.)
mp
18 °C (lit.)
density
0.855 g/mL at 25 °C (lit.)
application(s)
flavors and fragrances
Documentation
see Safety & Documentation for available documents
food allergen
no known allergens
Organoleptic
fatty; waxy
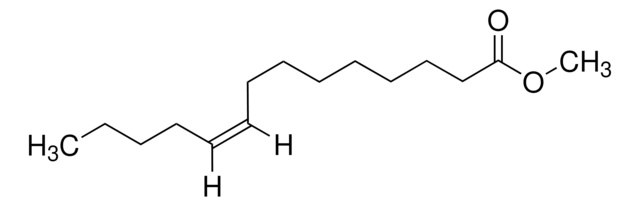
SMILES string
CCCCCCCCCCCCCC(=O)OC
InChI
1S/C15H30O2/c1-3-4-5-6-7-8-9-10-11-12-13-14-15(16)17-2/h3-14H2,1-2H3
InChI key
ZAZKJZBWRNNLDS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
It is one of the main:
- phytochemical constituent of Cycas Beddomei cones
- volatile flavor component of γ-aminobutyric acid (GABA) tea
Application
- Modulating the hydrophobicity of cellulose by lipase-catalyzed transesterification.: This research discusses the use of methyl myristate for modifying cellulose properties through enzymatic processes, which can be crucial for developing hydrophobic materials for various industrial applications (Sharma et al., 2024).
- Biosynthesis of insect sex pheromone precursors via engineered β-oxidation in yeast.: Demonstrates the synthesis of methyl myristate in yeast as a precursor to pheromones, suggesting its potential in bioengineering for sustainable agricultural practices (Petkevicius et al., 2022).
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
233.6 °F - closed cup
Flash Point(C)
112.0 °C - closed cup
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service