All Photos(1)
About This Item
Empirical Formula (Hill Notation):
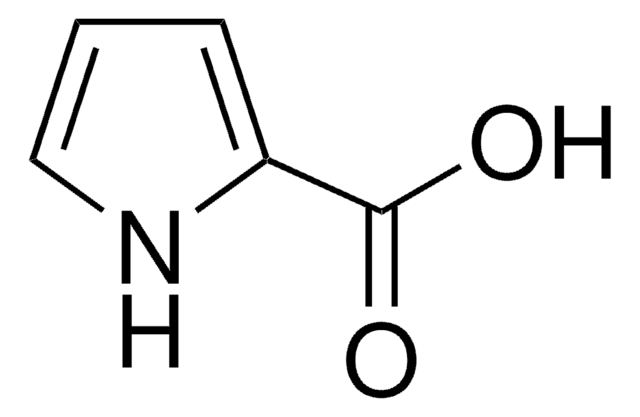
C5H4O2S
CAS Number:
Molecular Weight:
128.15
Beilstein:
110150
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
product line
ReagentPlus®
Assay
99%
bp
260 °C (lit.)
mp
125-127 °C (lit.)
SMILES string
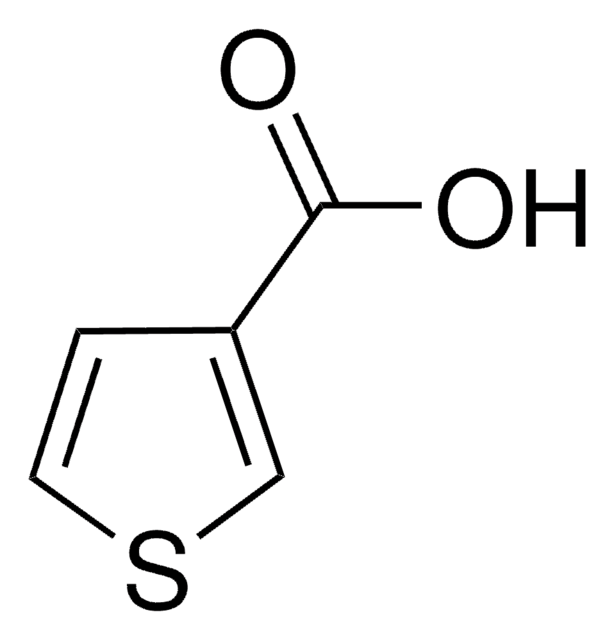
OC(=O)c1cccs1
InChI
1S/C5H4O2S/c6-5(7)4-2-1-3-8-4/h1-3H,(H,6,7)
InChI key
QERYCTSHXKAMIS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Not finding the right product?
Try our Product Selector Tool.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Mrinal K Bera et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 17(42), 11838-11843 (2011-09-08)
Herein, we describe our attempts to systematically prepare a series of oligo(2-thienyl)-substituted pyridine derivatives. The crucial starting material, a β-alkoxy-β-ketoenamide, is easily available on a large scale by the reaction of lithiated methoxyallene with thiophene-2-carbonitrile and thiophene-2-carboxylic acid. This three-component
Laval Chan et al.
Bioorganic & medicinal chemistry letters, 14(3), 793-796 (2004-01-27)
The discovery of a novel class of HCV NS5B polymerase inhibitors, 3-arylsulfonylamino-5-phenyl-thiophene-2-carboxylic acids is described. SAR studies have yielded several potent inhibitors of HCV polymerase as well as of HCV subgenomic RNA replication in Huh-7 cells.
J S Evans et al.
Applied microbiology and biotechnology, 32(6), 715-720 (1990-03-01)
A bacterium of the genus Vibrio, capable of degrading thiophene derivatives, was isolated from enrichment cultures inoculated with oil-contaminated estuarine mud. Of the thiophene derivatives tested, only thiophene-2-carboxylate (T2C), thiophene-2-acetate and thiophene-2-carbonyl chloride supported growth, but a total of seven
D Panagoulis et al.
Journal of inorganic biochemistry, 101(4), 623-634 (2007-02-06)
Copper complexes with thiophen-2-yl saturated and alpha,beta-unsaturated carboxylic acids as ligands were prepared, characterized and pharmacochemically studied. The available evidence supports a dimeric structure for the complexes of the general formula [Cu2(L)4(MeOH)2] where L are the anions of thiophene 2-carboxylic
Matthew F McCown et al.
Antimicrobial agents and chemotherapy, 53(5), 2129-2132 (2009-03-11)
In vitro, telaprevir selects subtype-specific resistance pathways for hepatitis C virus GT-1a and GT-1b, as described to have occurred in patients. In GT-1a, the HCV-796 resistance mutation C316Y has low replication capacity (7%) that can be compensated for by the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)