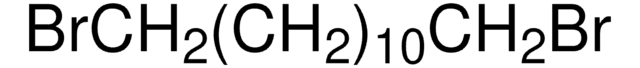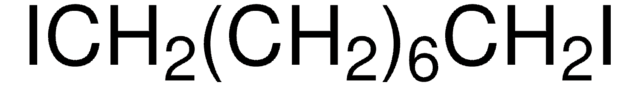D39800
1,10-Dibromodecane
97%
Synonym(s):
Decamethylene dibromide
About This Item
Recommended Products
Assay
97%
form
crystals
refractive index
n20/D 1.4912 (lit.)
bp
160 °C/15 mmHg (lit.)
mp
25-27 °C (lit.)
density
1.335 g/mL at 25 °C (lit.)
SMILES string
BrCCCCCCCCCCBr
InChI
1S/C10H20Br2/c11-9-7-5-3-1-2-4-6-8-10-12/h1-10H2
InChI key
GTQHJCOHNAFHRE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- as a reactant in the Wurtz-type reaction during the synthesis of polyethylene
- Construction of pillar[4]arene[1]quinone-1,10-dibromodecane pseudorotaxanes in solution and in the solid state.: This research highlights the application of 1,10-Dibromodecane in constructing complex molecular structures known as pseudorotaxanes. The study focuses on the synthesis and stabilization of these structures both in solution and solid states, providing a foundational technique for developing advanced materials in chemical engineering, pharmaceutical synthesis, and high-performance materials within industrial chemical manufacturing sectors. This breakthrough offers potential pathways for new drug delivery systems and smart materials based on the unique properties of these supramolecular assemblies (Sheng et al., 2020).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
233.6 °F - closed cup
Flash Point(C)
112 °C - closed cup
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service