803642
LC-SPDP (succinimidyl 6-[3(2-pyridyldithio)propionamido]hexanoate)
Synonym(s):
Long chain-SPDP, N-[6-[(2,5-dioxo-1-pyrrolidinyl)oxy]-6-oxohexyl]-3-(2-pyridinyldithio)propanamide
About This Item
Recommended Products
Assay
≥95%
Quality Level
form
powder
mol wt
425.52
reaction suitability
reagent type: cross-linking reagent
storage condition
desiccated
solubility
DMSO or DMF: soluble
functional group
NHS ester
shipped in
ambient
storage temp.
2-8°C
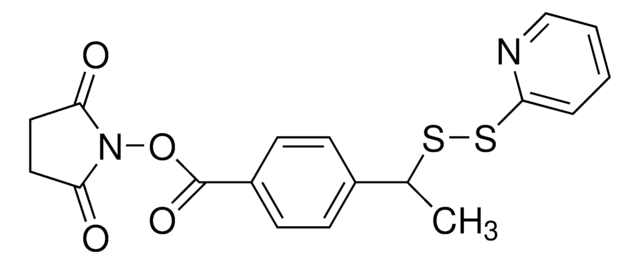
SMILES string
O=C1CCC(N1OC(CCCCCNC(CCSSC2=CC=CC=N2)=O)=O)=O
InChI
1S/C18H23N3O5S2/c22-14(10-13-27-28-15-6-3-5-12-20-15)19-11-4-1-2-7-18(25)26-21-16(23)8-9-17(21)24/h3,5-6,12H,1-2,4,7-11,13H2,(H,19,22)
InChI key
QYEAAMBIUQLHFQ-UHFFFAOYSA-N
General description
Features and Benefits
- Reactive groups: NHS ester and pyridyldithiol
- Reactive towards: amino and sulfhydryl groups
- Releases a detectable by-product when reacted with a free sulfhydryl group; by measuring the release of pyridine-2-thione at 343 nm, the reaction can be easily followed
- Disulfide bond in the spacer arm is readily cleaved by 10-50 mM DTT or TCEP at pH 8.5
- Spacer arm is also easily cleaved using reducing SDS-PAGE sample loading buffer
- Cleavable crosslinker allows separation of crosslinked products
- Water-insoluble (dissolve first in DMF or DMSO)
- SPDP crosslinker is membrane permeable, so crosslinking can be done inside the cells
- Compare to other varieties of SPDP-type reagents, including pegylated forms
Caution
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![Sulfo-LC-SPDP (sulfosuccinimidyl 6-[3′-(2-pyridyldithio)propionamido]hexanoate)](/deepweb/assets/sigmaaldrich/product/structures/266/633/e2a263be-4bd3-4fcf-89c4-75b5e2bd829c/640/e2a263be-4bd3-4fcf-89c4-75b5e2bd829c.png)