730114
2-Methacryloyloxyethyl phosphorylcholine
contains ≤100 ppm MEHQ as inhibitor, 97%
Synonym(s):
MPC
About This Item
Recommended Products
Assay
97%
form
powder or crystals
contains
≤100 ppm MEHQ as inhibitor
mp
143-148 °C
storage temp.
2-8°C
SMILES string
CC(=C)C(=O)OCCOP([O-])(=O)OCC[N+](C)(C)C
InChI
1S/C11H22NO6P/c1-10(2)11(13)16-8-9-18-19(14,15)17-7-6-12(3,4)5/h1,6-9H2,2-5H3
InChI key
ZSZRUEAFVQITHH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Use as a monomer to produce fully zwitterionic polymer networks in a solvate ionic liquid for use as a gel electrolyte in lithium-based batteries.
- Synthesis of polymer coatings for medical devices to prevent blood clotting and bacterial adhesion.
- Preparation of hydrogels for use as contact lens materials and wound dressing.
- Production of polymeric micelles and nanoparticles for drug delivery.
- Use as a monomer to prepare polymer-modified antifouling silicone hydrogel contact lenses. The addition of MPC forms a cell membrane-like structure on the contact lens and helps to prevent cell and bacterial adhesion on the surface.
Features and Benefits
- It has bioinert properties due to the presence of a zwitter ionic phosphorylcholine (PC) group in the side chain.
- It can build various molecular architectures with tunable properties.
- It has excellent resistance to cell adhesion, blood coagulation, and non-specific protein adsorption.
related product
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![[2-(Methacryloyloxy)ethyl]dimethyl-(3-sulfopropyl)ammonium hydroxide 95%](/deepweb/assets/sigmaaldrich/product/structures/217/219/73c91e1c-0ee4-4b3d-bead-a6dc3d09d1da/640/73c91e1c-0ee4-4b3d-bead-a6dc3d09d1da.png)

![[2-(Methacryloyloxy)ethyl]trimethylammonium chloride solution 75 wt. % in H2O](/deepweb/assets/sigmaaldrich/product/structures/316/612/66b0f4cf-d060-427d-b4f5-e8fab3e5cffe/640/66b0f4cf-d060-427d-b4f5-e8fab3e5cffe.png)
![Bis[2-(methacryloyloxy)ethyl] phosphate](/deepweb/assets/sigmaaldrich/product/structures/128/336/4e7a3e38-338c-423e-95b8-70d9d1f8e121/640/4e7a3e38-338c-423e-95b8-70d9d1f8e121.png)
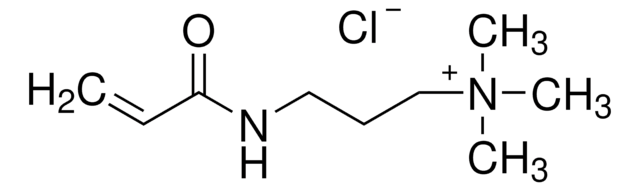

![[2-(Acryloyloxy)ethyl]trimethylammonium chloride solution 80 wt. % in H2O, contains 600 ppm monomethyl ether hydroquinone as inhibitor](/deepweb/assets/sigmaaldrich/product/structures/393/326/f7e19585-5431-4220-81b5-f458de6d63d0/640/f7e19585-5431-4220-81b5-f458de6d63d0.png)




