All Photos(1)
About This Item
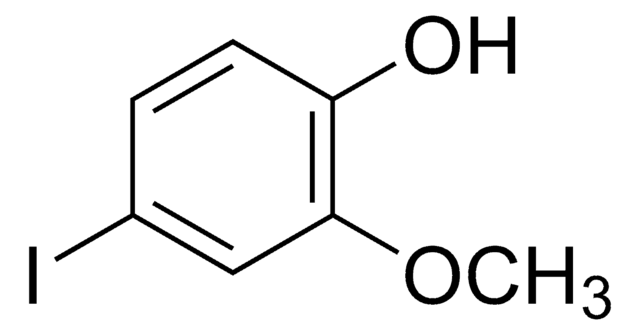
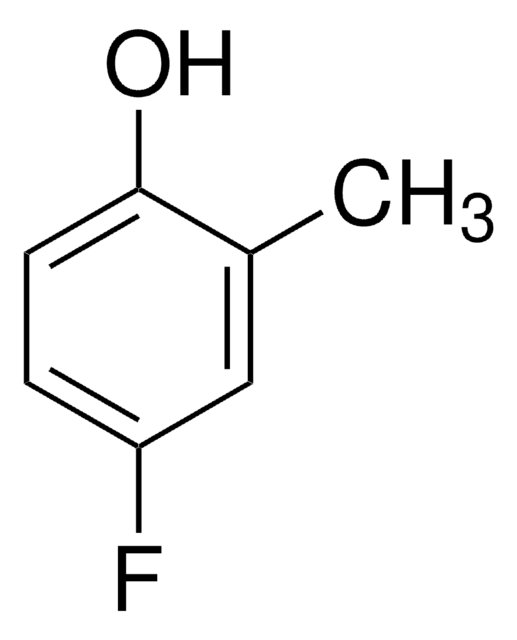
Linear Formula:
FC6H3(OCH3)OH
CAS Number:
Molecular Weight:
142.13
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
refractive index
n20/D 1.517 (lit.)
bp
195 °C (lit.)
density
1.247 g/mL at 25 °C (lit.)
SMILES string
COc1cc(F)ccc1O
InChI
1S/C7H7FO2/c1-10-7-4-5(8)2-3-6(7)9/h2-4,9H,1H3
InChI key
OULGLTLTWBZBLO-UHFFFAOYSA-N
General description
4-Fluoro-2-methoxyphenol is a fluorinated methoxy-substituted catechol analog.
Application
4-Fluoro-2-methoxyphenol may be used in the synthesis of:
- 4-halo-masked o-benzoquinones (MOBs)
- fluorinated masked o-benzoquinone
- poly(4-fluoro-2-methoxyphenol)
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
214.9 °F - closed cup
Flash Point(C)
101.6 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Novel photoconductive polyfluorophenol synthesized by an enzyme.
Zaragoza-Gasca P, et al.
Journal of Molecular Catalysis. B, Enzymatic, 72(1), 25-27 (2011)
Diels-Alder reactions of halogenated masked o-benzoquinones: synthesis of halogen-substituted bicyclo [2.2. 2] octenones.
Tetrahedron Letters, 50(7), 773-775 (2009)
A fluorinated masked o-benzoquinone.
Patrick TB, et al.
Journal of Fluorine Chemistry, 125(12), 1965-1966 (2007)
P K Chakraborty et al.
International journal of radiation applications and instrumentation. Part A, Applied radiation and isotopes, 42(7), 673-681 (1991-01-01)
The synthesis of 4-[18F]fluoroguaiacol (4-[18F]fluoro-2-methoxyphenol) has been achieved in no-carrier-added form starting from 2-methoxy-4-nitrobenzaldehyde, using nucleophilic aromatic substitution by [18F]fluoride followed by Baeyer-Villiger oxidation of the benzaldehyde to the phenol. Demethylation with boron tribromide gave 4-[18F]fluorocatechol (1,2-dihydroxy-4-[18F]fluorobenzene) with an overall
Ana Carolina de Almeida et al.
European journal of pharmacology, 660(2-3), 445-453 (2011-04-19)
Apocynin, a methoxy-substituted catechol (4-hydroxy-3-methoxyacetophenone), originally extracted from the roots of Picrorhiza kurroa, has been extensively used as a non-toxic inhibitor of the multienzymatic complex NADPH oxidase. We discovered that the analogous methoxy-substituted catechol, 4-Fluoro-2-methoxyphenol (F-apocynin), in which the acetyl
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service