All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H3ClIN
CAS Number:
Molecular Weight:
239.44
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
95-98 °C (lit.)
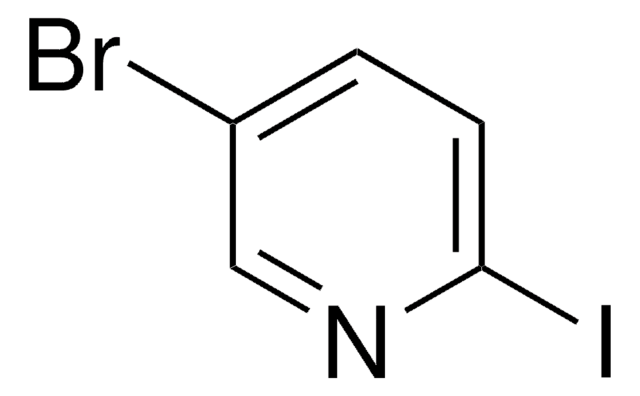
SMILES string
Clc1ccc(I)cn1
InChI
1S/C5H3ClIN/c6-5-2-1-4(7)3-8-5/h1-3H
InChI key
QWLGCWXSNYKKDO-UHFFFAOYSA-N
General description
2-Chloro-5-iodopyridine is a halo-substituted pyridine.
Application
2-Chloro-5-iodopyridine may be used as a reagent in the multi-step synthesis of (±)-epibatidine.
It may be used in the synthesis of:
It may be used in the synthesis of:
- 2-Chloro-5-phenylpyridine via Suzuki coupling reaction with phenylboronic acid dimethyl ester.
- Exo-5- and exo-6- (6′-chloro-3′-pyridyl)-2-azabicyclo[2.2.1]heptanes via Heck coupling reaction with N-protected 2-azabicyclo[2.2.1]hept-5-enes.
- Substituted diaryliodonium salts.
- 3-Exo-5′-(2′-Chloropyridinyl)-8-(ethoxycarbonyl)-8-azabicyclo[3.2.1]octane.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
High-yielding one-pot synthesis of diaryliodonium triflates from arenes and iodine or aryl iodides.
Bielawski M and Olofsson B
Chemical Communications (Cambridge, England), 24, 2521-2523 (2007)
A short and efficient total synthesis of (?)-epibatidine.
A short and efficient total synthesis of (?)-epibatidine.
A short and efficient total synthesis of (?)-epibatidine.
Zhang C and Trudell ML.
The Journal of Organic Chemistry, 61(20), 7189-7191 (1996)
Syntheses of new open-ring and homo-epibatidine analogues from tropinone.
Olivo HF, et al.
The Journal of Organic Chemistry, 64(13), 4966-4968 (1999)
Synthesis of epibatidine isomers: Reductive Heck coupling of 2-azabicyclo [2.2.1] hept-5-ene derivatives.
Cox CD and Malpass JR
Tetrahedron, 55(40), 11879-11888 (1999)
Synthesis of 5-Substituted 2,2'-Bipyridines from Substituted 2-Chloropyridines by a Modified Negishi Cross-Coupling Reaction.
Lutzen A and Hapke M.
European Journal of Organic Chemistry, 2002(14), 2292-2297 (2002)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)