412244
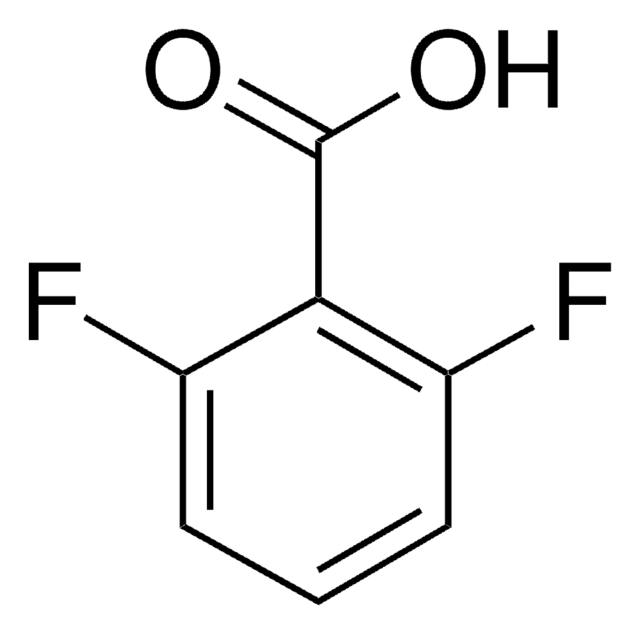
2-Fluorobenzoic acid
97%
Synonym(s):
o-Fluorobenzoic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
FC6H4CO2H
CAS Number:
Molecular Weight:
140.11
Beilstein:
971265
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
122-125 °C (lit.)
SMILES string
OC(=O)c1ccccc1F
InChI
1S/C7H5FO2/c8-6-4-2-1-3-5(6)7(9)10/h1-4H,(H,9,10)
InChI key
NSTREUWFTAOOKS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
2-Fluorobenzoic acid may be employed in the preparation of zaragozic acid A analogs.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
S Y Lee et al.
Nuclear medicine and biology, 28(4), 391-395 (2001-06-08)
In vitro metabolism of acetylcholinesterase inhibitors containing 3-[(18)F]fluoromethylbenzyl- ([(18)F]1) and 4-[(18)F]fluorobenzyl-piperidine moieties ([(18)F]2) was studied and compared with the in vivo metabolism. Defluorination of the [(18)F]1 mainly occurred to generate [(18)F]fluoride ion both in vitro and in vivo. In contrast
J Toretsky et al.
Nuclear medicine and biology, 31(6), 747-752 (2004-07-13)
The clinical response to antitumor therapy is measured using imaging, such as CT or MRI, 6-12 weeks following chemotherapy treatment. The images at that time reflect both tumor cell death and new growth. Therefore, the amount of tumor cell death
Microbial degradation of synthetic organochlorine compounds.
K Motosugi et al.
Experientia, 39(11), 1214-1220 (1983-11-15)
U Schennen et al.
Journal of bacteriology, 161(1), 321-325 (1985-01-01)
Three strains of anaerobically benzoate-degrading, denitrifying bacteria of the genus Pseudomonas were able to grow on 2-fluorobenzoate as the sole carbon and energy source. Fluoride ion release was stoichiometric, and the reduction of dissolved organic carbon indicated total degradation. Cells
T S Chen et al.
The Journal of antibiotics, 47(11), 1290-1294 (1994-11-01)
Zaragozic acid A analogues are produced by an unidentified sterile fungus when it is exogenously supplied with 2-thiophenecarboxylic acid, 3-thiophenecarboxylic acid, 2-furoic acid, 2-fluorobenzoic acid, 3-fluorobenzoic acid, or 4-fluorobenzoic acid. The analogues carry 2-thiophenyl, 3-thiophenyl, 2-furyl, o-fluorophenyl, m-fluorophenyl, or p-fluorophenyl
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service