388513
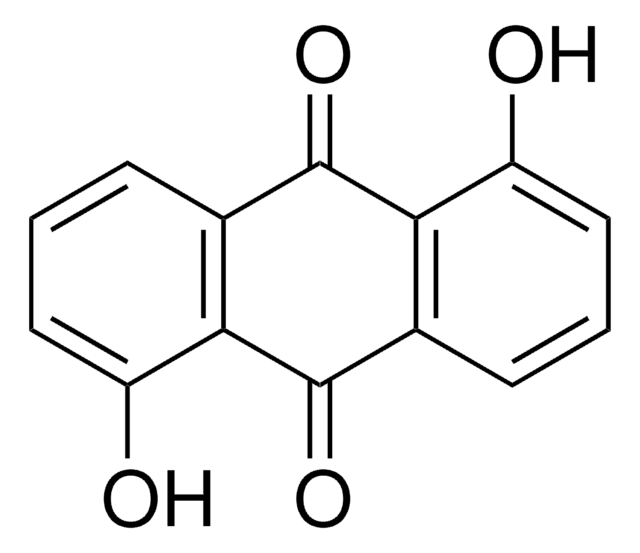
5,8-Dihydroxy-1,4-naphthoquinone
technical grade
Synonym(s):
Naphthazarin
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H6O4
CAS Number:
Molecular Weight:
190.15
Beilstein:
880561
EC Number:
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
grade
technical grade
mp
220-230 °C (lit.)
SMILES string
Oc1ccc(O)c2C(=O)C=CC(=O)c12
InChI
1S/C10H6O4/c11-5-1-2-6(12)10-8(14)4-3-7(13)9(5)10/h1-4,11-12H
InChI key
RQNVIKXOOKXAJQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Brígida R Pinho et al.
PloS one, 6(8), e24098-e24098 (2011-09-03)
The search of new anti-inflammatory drugs has been a current preoccupation, due to the need of effective drugs, with less adverse reactions than those used nowadays. Several naphthoquinones (plumbagin, naphthazarin, juglone, menadione, diosquinone and 1,4-naphthoquinone), plus p-hydroquinone and p-benzoquinone were
Régis Millet et al.
Journal of medicinal chemistry, 48(22), 7024-7039 (2005-10-28)
The human selenoprotein thioredoxin reductase is involved in antioxidant defense and DNA synthesis. As increased thioredoxin reductase levels are associated with drug sensitivity to cisplatin and drug resistance in tumor cells, this enzyme represents a promising target for the development
An efficient formal synthesis of the human telomerase inhibitor (+/-)-gamma-rubromycin.
Dominea C K Rathwell et al.
Angewandte Chemie (International ed. in English), 48(43), 7996-8000 (2009-08-01)
C J Yong-Kee et al.
Neurotoxicity research, 22(4), 355-364 (2012-04-25)
Delineation of how cell death mechanisms associated with Parkinson's disease (PD) interact and whether they converge would help identify targets for neuroprotective therapies. The purpose of this study was to use a cellular model to address these issues. Catecholaminergic SH-SY5Y
Christina L L Chai et al.
The Journal of organic chemistry, 71(3), 992-1001 (2006-01-28)
A concise formal total synthesis of the cytotoxic bisnaphthazarin derivative hybocarpone has been completed through the development of routes to the synthetic precursor, 3-ethyl-2-hydroxy-5,7,8-trimethoxy-6-methyl-1,4-naphthoquinone. The oxidation of 3-ethyl-1,2,4,5,7,8-hexamethoxy-6-methylnaphthalene under Rapoport conditions gave 3-ethyl-2-hydroxy-5,7,8-trimethoxy-6-methyl-1,4-naphthoquinone in modest yields after basic hydrolysis. In
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service