All Photos(1)
About This Item
Empirical Formula (Hill Notation):
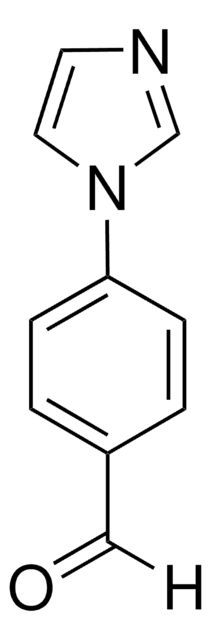
C11H10N2O
CAS Number:
Molecular Weight:
186.21
EC Number:
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
form
solid
mp
108-110 °C (lit.)
SMILES string
CC(=O)c1ccc(cc1)-n2ccnc2
InChI
1S/C11H10N2O/c1-9(14)10-2-4-11(5-3-10)13-7-6-12-8-13/h2-8H,1H3
InChI key
GAIQQJIMVVUTQN-UHFFFAOYSA-N
General description
4′-(Imidazol-1-yl)acetophenone is a selective thromboxane synthesis inhibitor.
Application
4′-(Imidazol-1-yl)acetophenone was used in the synthesis of (R)-(+)-4′-(imidozol-1-yl)-phenyl ethanol using spiroborate catalyst.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A J Watson et al.
American journal of kidney diseases : the official journal of the National Kidney Foundation, 8(1), 26-30 (1986-07-01)
It has recently been postulated that thromboxane A2 may participate in the pathogenesis of acute myohemoglobinuric experimental acute renal failure. To investigate this further, the effect of selective inhibition of thromboxane synthesis on the course of glycerol-induced acute renal failure
J Triscari et al.
International journal of obesity, 11 Suppl 3, 43-51 (1987-01-01)
A selective inhibitor of thromboxane synthase, Ro 22-3581 has been shown to be a useful tool for investigating the relationship between hyperinsulinemia and obesity. These studies have established that the pharmacologic normalization of the hyperinsulinemia associated with elevated weights in
Viatcheslav Stepanenko et al.
Tetrahedron, asymmetry, 18(23), 2738-2745 (2007-11-26)
The effectiveness of several spiroborate ester catalysts was investigated in the asymmetric borane reduction of 2-, 3-, 4-acetylpyridines under different reaction conditions. Highly enantiomerically enriched 1-(2-, 3- and 4-pyridyl)ethanols and 1-(heterocyclic)ethanols were obtained using 1 to 10% catalytic loads of
H D Uderman et al.
Prostaglandins, 24(2), 237-244 (1982-08-01)
The compound 4'-(imidazol-1-yl) acetophenone was demonstrated to be a selective thromboxane (Tx) synthetase inhibitor in spontaneously hypertensive rats (SHR). Serum TxB2 concentrations (from clotted blood) were suppressed by 89.1% (p less than 0.001) and 41.2% (p less than 0.01) at
W D Watkins et al.
Prostaglandins, 23(3), 273-285 (1982-03-01)
We assessed the effect of a specific thromboxane synthetase inhibitor (an imidazole derivative) on pulmonary hemodynamics and the concentrations of TxB2 (TxA2), 6-keto-PGF1 alpha (PGI2), and PGF2 in pulmonary lymph and transpulmonary blood samples following intravenous administration of E. coli
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service