158348
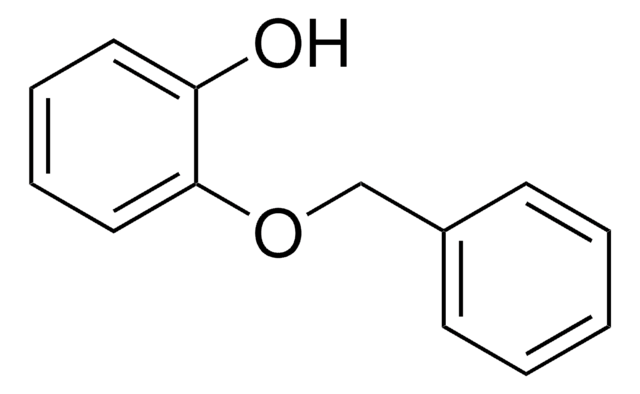
4-(Benzyloxy)phenol
98%
Synonym(s):
4-(Phenylmethoxy)phenol, Hydroquinone monobenzyl ether
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5CH2OC6H4OH
CAS Number:
Molecular Weight:
200.23
Beilstein:
1958305
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
119-120 °C (lit.)
functional group
phenyl
SMILES string
Oc1ccc(OCc2ccccc2)cc1
InChI
1S/C13H12O2/c14-12-6-8-13(9-7-12)15-10-11-4-2-1-3-5-11/h1-9,14H,10H2
InChI key
VYQNWZOUAUKGHI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
4-(Benzyloxy)phenol was used in the synthesis of bis(4-benzyloxyphenoxy)phenyl phosphine oxide. It was also used in the preparation of series of hetaryl-azophenol dyes via heterocyclic amines in nitrosyl sulphuric acid.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis, spectral properties, biological activity and application of new 4-(benzyloxy) phenol derived azo dyes for polyester fiber dyeing.
Yousefi H, et al.
Journal of Molecular Liquids, 180, 51-58 (2013)
K M AlGhamdi et al.
Journal of the European Academy of Dermatology and Venereology : JEADV, 25(7), 749-757 (2010-11-09)
If vitiligo involves most of the body, it might be easier to depigment the normal remaining skin rather than to attempt repigmentation. We reviewed the literature to date regarding available therapies for depigmenting the normal skin in vitiligo universalis. Our
K Urabe et al.
Seminars in cutaneous medicine and surgery, 16(1), 81-85 (1997-03-01)
The dyschromatoses are a group of disorders characterized by the presence of both hyperpigmented and hypopigmented macules, many of which are small in size and irregular in shape. There are two major forms-dyschromatosis symmetrica hereditaria (DSH) and dyschromatosis universalis hereditaria
Jasper G van den Boorn et al.
Pigment cell & melanoma research, 24(4), 673-679 (2011-06-22)
Autoimmune side-effects such as vitiligo regularly occur during melanoma immunotherapy. As vitiligo development is associated with a superior prognosis, the active induction of vitiligo in melanoma patients can be a useful tactic. The potent skin-depigmenting agent monobenzone can be used
E Frenk
Der Hautarzt; Zeitschrift fur Dermatologie, Venerologie, und verwandte Gebiete, 37(1), 1-5 (1986-01-01)
The treatment of vitiligo is still unsatisfactory in many patients. The only two methods that have been evaluated in numerous patients are classic psoralen-light therapy and local treatment with potent fluorinated corticosteroids. These two methods in addition to some more
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service