All Photos(1)
About This Item
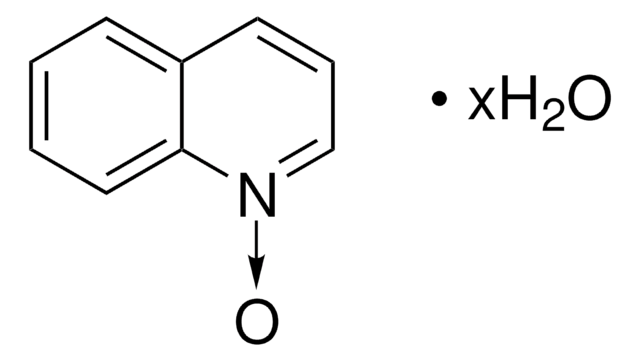
Empirical Formula (Hill Notation):
C5H5NO
CAS Number:
Molecular Weight:
95.10
Beilstein:
105257
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
form
solid
bp
270 °C (lit.)
mp
62-67 °C (lit.)
SMILES string
[O-][n+]1ccccc1
InChI
1S/C5H5NO/c7-6-4-2-1-3-5-6/h1-5H
InChI key
ILVXOBCQQYKLDS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Pyridine N-oxide axle with [2]rotaxanes was synthesized via an anion templated threading-followed-by-stoppering strategy.
Application
Pyridine N-oxide was used to study the FTIR spectra of pyridine N-oxide in acetonitrile.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
289.4 °F - closed cup
Flash Point(C)
143 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Highly efficient gold nanoparticle catalyzed deoxygenation of amides, sulfoxides, and pyridine N-oxides.
Yusuke Mikami et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 17(6), 1768-1772 (2011-01-29)
Jan Balzarini et al.
The Journal of antimicrobial chemotherapy, 55(2), 135-138 (2005-01-15)
Pyridine N-oxide derivatives represent a new class of anti-HIV compounds, for which some members exclusively act through inhibition of HIV-1 reverse transcriptase and thus characteristically behave as non-nucleoside reverse transcriptase inhibitors. Other members act, additionally or alternatively, at a post-integrational
G Pitsevich et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 120, 585-594 (2014-01-01)
FTIR spectra of pyridine N-oxide and trichloroacetic acid H-bonded complex in acetonitrile were studied at 20 and 50°C. The calculations of equilibrium configurations of the complex and their IR spectra in harmonic- and anharmonic approximations were carried out at the
Masahito Murai et al.
Chemical communications (Cambridge, England), 48(61), 7622-7624 (2012-06-26)
Gold(I)-catalysed tandem oxygen-transfer/cycloisomerisation reaction of 2-(2-propynyl)pyridine N-oxides provides an atom-economical route to indolizinone frameworks.
Xue Gong et al.
Organic letters, 13(7), 1766-1769 (2011-03-11)
A Pd(II)-catalyzed oxidative coupling between pyridine N-oxides and N-substituted indoles via 2-fold C-H bond activation was achieved with high selectivity using Ag(2)CO(3) as an oxidant.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service