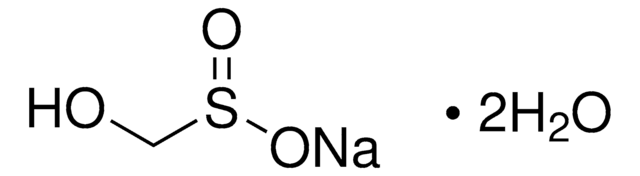112704
Formaldehyde-sodium bisulfite adduct
95%
Synonym(s):
Sodium formaldehyde bisulfite
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Linear Formula:
HOCH2SO3Na
CAS Number:
Molecular Weight:
134.09
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
solid
mp
200 °C (dec.) (lit.)
SMILES string
O=S(CO)([O-])=O.[Na+]
InChI
1S/CH4O4S.Na/c2-1-6(3,4)5;/h2H,1H2,(H,3,4,5);/q;+1/p-1
InChI key
UOULCEYHQNCFFH-UHFFFAOYSA-M
Related Categories
General description
Formaldehyde-sodium bisulfite adduct is used as a standard in the synthesis of 3-Hydroxypropionaldehyde (3HPA). It can be assayed by HPLC with fluorometric detection in selected foods.
Application
- Triethyl citrate as a non-toxic solvent in pharmaceutical applications: Explored the use of Triethyl citrate in in-situ forming PLGA implants, focusing on its role as a less toxic solvent compared to traditional options. This application demonstrates the importance of Triethyl citrate in developing safer pharmaceutical formulations (Ramos et al., 2024).
- Triethyl citrate in biodegradable polymer research: Examines the influence of Triethyl citrate on the melt spinning and structural properties of poly(lactic acid) fibers. This study highlights its effectiveness as a plasticizer, enhancing the processability and properties of biodegradable polymers, relevant for controlled drug release systems (Gzyra-Jagieła et al., 2024).
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yuegang Zuo
Chemosphere, 51(3), 175-179 (2003-02-20)
The determination of the photo-production rate of hydroxyl radical (OH) in atmospheric liquids is of fundamental importance to an understanding of atmospheric aquatic chemistry. Recently, several studies have been performed to examine the photo-chemical formation rate of OH in cloud
R Aris et al.
The American review of respiratory disease, 141(3), 546-551 (1990-03-01)
Hydroxymethanesulfonate (HMSA), the bisulfite (HSO3-) adduct of formaldehyde (CH2O), is a common constituent of California acid fogs. HMSA, most stable in a fog pH range of 3 to 5, dissociates at 6.6, the pH of the fluid lining human airways.
C R Warner et al.
Food additives and contaminants, 7(5), 575-581 (1990-09-01)
The reaction of sulphite with formaldehyde to form hydroxymethylsulphonate (HMS), which is very stable under the controlled conditions of this assay, was used as the first step in an analytical procedure to determine foodborne sulphite. The effect of mobile-phase pH
Stephen Wai Cheung Chung et al.
Journal of AOAC International, 91(1), 98-102 (2008-04-02)
A rapid and accurate method for measuring low part-per-million levels of free and reversibly-bound sulfites in selected foods by using high-performance liquid chromatography (HPLC) with fluorometric detection was developed. Sulfites were extracted with sodium tetrachloromercurate solution and determined by HPLC-fluorescence
Hydroxymethylated polysulphone for islet macroencapsulation allows rapid diffusion of insulin but retains PERV.
P Petersen et al.
Transplantation proceedings, 34(1), 194-195 (2002-04-18)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service