All Photos(2)
About This Item
Linear Formula:
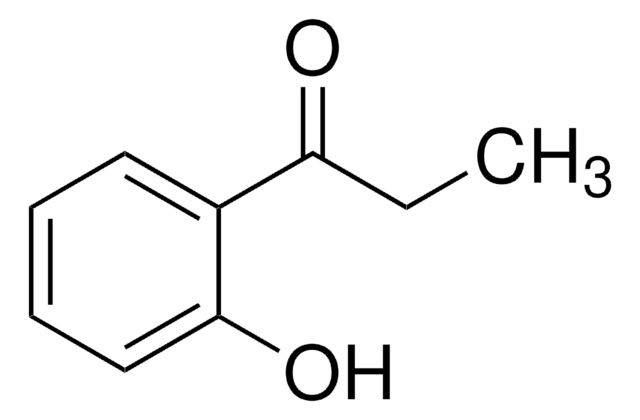
C6H5COCH2OH
CAS Number:
Molecular Weight:
136.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
86-89 °C (lit.)
SMILES string
OCC(=O)c1ccccc1
InChI
1S/C8H8O2/c9-6-8(10)7-4-2-1-3-5-7/h1-5,9H,6H2
InChI key
ZWVHTXAYIKBMEE-UHFFFAOYSA-N
Application
2-Hydroxyacetophenone can be used as a starting material for the synthesis of:
- Enantioselective 1R-phenyl-1,2-ethanediol in the presence of a rhodium(III) catalyst by asymmetric transfer hydrogenation.
- Copper(II) complexes of 2-hydroxyacetophenone N-substituted thiosemicarbazones.
- Chromium, molybdenum, and ruthenium complexes of 2-hydroxyacetophenone Schiff bases.
- 2-Hydroxyacetophenone-aroyl hydrazone derivatives for inhibition of copper corrosion in nitric acid.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ying-Heng Chen et al.
Journal of agricultural and food chemistry, 65(19), 3965-3974 (2017-04-30)
4-[2-(t-Butylamino)-1-hydroxyethyl]phenol (buctopamine, 4), a new β
Resonance Raman intensity analysis of the excited-state proton transfer in 2-hydroxyacetophenone.
Peteanu LA and Mathies RA.
The Journal of Physical Chemistry, 96(17), 6910-6916 (1992)
Na Li et al.
Molecules (Basel, Switzerland), 25(14) (2020-07-28)
Fluorophores with aggregation-induced emission enhancement (AIEE) characteristics applied in bioimaging have attracted more and more attention in recent years. In this work, a series of flavanone compounds with AIEE characteristics was developed and applied to fluorescence imaging of mitochondria and
Chromium, molybdenum and ruthenium complexes of 2-hydroxyacetophenone Schiff bases
Ali SA, et al.
Journal of Coordination Chemistry, 55(10), 1161-1170 (2002)
Synthetic studies on optically active Schiff-base ligands derived from condensation of 2-hydroxyacetophenone and chiral diamines.
Gao WT and Zheng Z.
Molecules (Basel), 7(7), 511-516 (2002)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service