148113
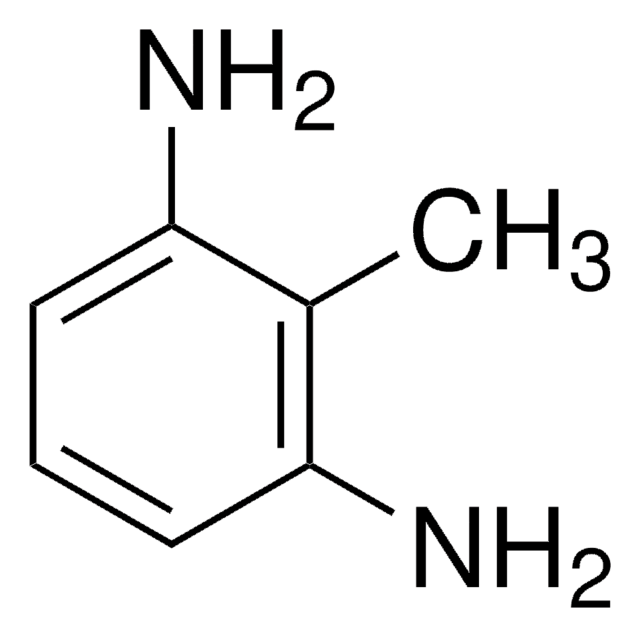
2,6-Diaminotoluene
97%
Synonym(s):
2,6-Toluenediamine, 2,6-Tolylenediamine, 2-Methyl-m-phenylenediamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3C6H3(NH2)2
CAS Number:
Molecular Weight:
122.17
Beilstein:
2079476
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
97%
form
solid
mp
104-106 °C (lit.)
SMILES string
Cc1c(N)cccc1N
InChI
1S/C7H10N2/c1-5-6(8)3-2-4-7(5)9/h2-4H,8-9H2,1H3
InChI key
RLYCRLGLCUXUPO-UHFFFAOYSA-N
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Aquatic Chronic 2 - Muta. 2 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M L Cunningham et al.
Environmental health perspectives, 104 Suppl 3, 683-686 (1996-05-01)
The aromatic amines 2,4-diaminotoluene (2,4-DAT) and 2,6-diaminotoluene (2,6-DAT) are structural isomers that have been extensively studied for their mutagenic and carcinogenic characteristics. Both compounds are rapidly absorbed after oral administration and are equally mutagenic in the Ames test; however, 2,4-DAT
Differential in vivo mutagenicity of the carcinogen/non-carcinogen pair 2,4- and 2,6-diaminotoluene.
J J Hayward et al.
Carcinogenesis, 16(10), 2429-2433 (1995-10-01)
The aromatic amines 2,4-diaminotoluene (2,4-DAT) and 2,6-diaminotoluene (2,6-DAT) are structural isomers that have been extensively studied for their mutagenic and carcinogenic characteristics. Both compounds are equally mutagenic in the Ames/Salmonella assay in the presence of S9. However, the differences in
P Lind et al.
The Analyst, 122(1), 51-56 (1997-01-01)
Blood and urine samples were collected from six workers and two volunteers exposed to thermal degradation products from toluene diisocyanate (TDI)-based polyurethane (PUR) before and during the summer vacation. Air samples were collected on filters impregnated with 9-(N-methylaminomethyl)anthracene. The concentrations
Diaminotoluenes induce intrachromosomal recombination and free radicals in Saccharomyces cerevisiae.
R J Brennan et al.
Mutation research, 381(2), 251-258 (1998-01-22)
The carcinogenicity of aniline-based aromatic amines is poorly reflected by their activity in short-term mutagenicity assays such as the Salmonella typhimurium reverse mutation (Ames) assay. More information about the mechanism of action of such carcinogens is needed. Here we report
M Taningher et al.
Toxicology, 99(1-2), 1-10 (1995-05-05)
Among aminoaromatics, 2,4-diaminotoluene (2,4-DAT) and 2,6-diaminotoluene (2,6-DAT) represent a conflicting couple of isomers; despite showing the same structural alert to DNA reactivity (and thus potential genotoxicity), they are different in terms of carcinogenicity. Of the two, 2,4-DAT alone is a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service