おすすめの製品
product name
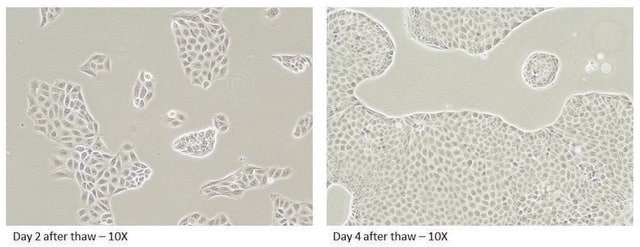
MDR1 Knockout HepaRG™ Cells, 1 vial
由来生物
human female liver (Source Disease: Hepatocarcinoma and Hepatitis C)
形状
liquid
保管温度
−196°C
遺伝子情報
human ... ABCB1(5243)
詳細
アプリケーション
特徴および利点
- Sigma′s HepaRG MDR1 Knockout (KO) allows investigations of drug-transporter interactions involving MDR1 (P-glycoprotein) in the liver.
- The frame-shift mutation of MDR1 gene was confirmed by fragment length analysis and DNA sequencing.
- Loss of functionality was confirmed by loss of transport of selective substrates in sandwich culture assay.
品質
法的情報
免責事項
保管分類コード
12 - Non Combustible Liquids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
MTOX1019-1VL:
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
資料
Oral drug delivery involves dissolution in the small intestine and absorption across the enterocyte barrier into the portal vein followed by subsequent delivery through the liver into the systemic circulation.
経口薬の送達には、小腸での溶解、腸細胞のバリアを超えた門脈への吸収、それに続く肝臓を介した体循環への送達が関わっています。
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)