おすすめの製品
形状
solid
光学活性
[α]22/D −64°, c = 7 mg/mL in methanol(lit.)
色
light brown
溶解性
alcohol: soluble (Solutions should be freshly prepared.)
aqueous acid: moderately soluble (Solutions should be freshly prepared)
aqueous base: insoluble
保管温度
2-8°C
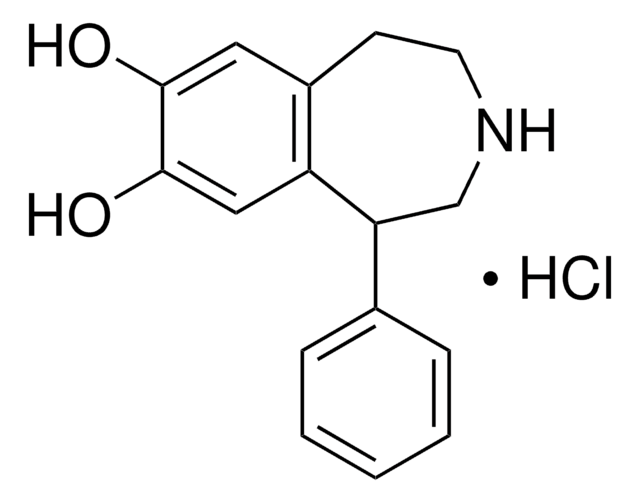
SMILES記法
Br.[H][C@]12Cc3ccc(O)c(O)c3-c4cc(O)cc(CCN1CCC)c24
遺伝子情報
human ... DRD2(1813)
生物化学的/生理学的作用
Potent, selective D2 dopamine receptor agonist.
保管分類コード
13 - Non Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
個人用保護具 (PPE)
Eyeshields, Gloves, type N95 (US)
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
E A Jackson et al.
European journal of pharmacology, 87(1), 15-23 (1983-01-28)
The behavioral actions of some novel aporphines have been examined in rats with selective unilateral 6-hydroxydopamine (6OHDA)-induced destruction of nigrostriatal dopaminergic neurons, and in rats with bilateral 6OHDA-induced destruction of mesolimbic dopaminergic neurons. Dopaminomimetics such as apomorphine (APO) in these
Y G Gao et al.
Journal of medicinal chemistry, 33(6), 1800-1805 (1990-06-01)
Syntheses of (R)-(-)-2-methoxyapomorphine (R-8), its antipode S-8, and its (R)-(-)-N-n-propyl R-9 derivatives are described. The dopaminergic receptor affinities of these compounds and their 2-unsubstituted counterparts (R)-(-)-apomorphine (R(-)-APO, R-1), (S)-(+)-apomorphine (S(+)-APO, S-1), and (R)-(-)-N-n-propylnorapomorphine (R(-)-NPA, R-2), as well as those of
E Chu et al.
Experimental eye research, 69(6), 611-616 (2000-01-06)
The purpose of this study was to correlate potential mechanisms with site(s) of action for TNPA-induced ocular hypotension. In response to R(-)-2, 10, 11-trihydroxy-N-propyl-noraporphine hydrobromide (TNPA, 75 microg), a D2 dopamine receptor agonist, the intraocular pressure decreased by 4.5 and
J L Neumeyer et al.
Journal of medicinal chemistry, 24(7), 898-899 (1981-07-01)
(-)-2,10,11-Trihydroxy-N-n-propylnoraporphine (TNPA,2c) has been synthesized from thebaine (3a), via northebaine (3b), normorphothebaine (2a), and alkylation to the N-propyl derivative 2b. O-Demethylation gave the desired product 2c. Compound 2c showed activity comparable to its 10,11-dihyroxy counterpart (NPA, 1b) on the stimulation
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)