5.32283
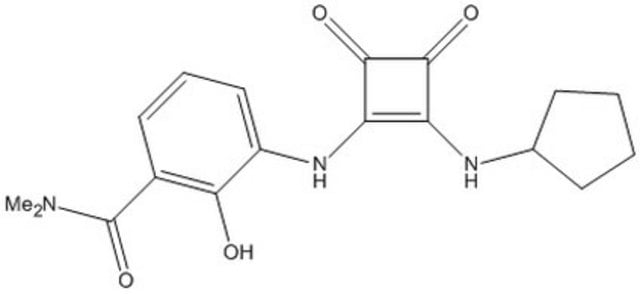
CXCR2 Antagonist IV, Sch527123
別名:
CXCR2 Antagonist IV, Sch527123, 2-Hydroxy-N,N-dimethyl-3-((2-((1( R)-(5-methyl-2-furanyl)propyl)amino)-3,4-dioxo-1-cyclobuten-1-yl)amino)benzamide, ( R)-2-Hydroxy-N,N-dimethyl-3-(2-(1-(5-methylfuran-2-yl)propylamino)-3,4-dioxocyclobut-1-enylamino)benzamide, CXCR1 Antagoni
ログイン組織・契約価格を表示する
すべての画像(1)
About This Item
おすすめの製品
アッセイ
≥97% (HPLC)
品質水準
形状
solid
メーカー/製品名
Calbiochem®
保管条件
OK to freeze
protect from light
色
yellow
溶解性
DMSO: 50 mg/mL
保管温度
2-8°C
InChI
1S/C21H23N3O5/c1-5-13(15-10-9-11(2)29-15)22-16-17(20(27)19(16)26)23-14-8-6-7-12(18(14)25)21(28)24(3)4/h6-10,13,22-23,25H,5H2,1-4H3/t13-/m1/s1
InChI Key
RXIUEIPPLAFSDF-CYBMUJFWSA-N
詳細
A cell-permeable orally available (plasma Cmax/AUC/dose = 17.0 µM/31.5 µM h-10-24 h/25 mg kg-1/mouse; 5.0 µM/23.0 µM h-10-48 h/10 mg kg-1/rat; 0.9 µM/4.0 µM h-10-48 h/3 mg kg-1/Cynomolgus monkey) cyclobutenedione compound that targets CXCR2 intracellular allosteric site via high affinity interaction (Kd = 49 pM/human, 80 pM/Cynomolgus monkey, 200 pM/ mouse & rat) in a reversible and slowly dissociating manner (k2 = 0.00058 min-1; t1/2 ≈ 22 h; human CXCR2), while exhibiting much reduced affinity toward CXCR1 (Kd = 3.9 nM/human & 41 nM/Cynomolgus monkey) due to a much faster dissociation rate (k2 = 0.141 min-1; t1/2 ≈ 5 min; human CXCR1) and displaying no significant efficacy toward a large panel of GPCRs, enzymes, and ion channels (IC50 >10 µM), including CXCR3 & CCR5. Likewise, Sch527123 is shown exhibit much higher potency against CXCR2- than CXCR1-mediated chemotaxis of Ba/F3 transfectants (IC50/ligand/CXCR2 species = 2.8 nM/3 nM hCXCL8/monkey, 3.4 nM/3 nM rCINC-3/rat, 5.6 nM/mCXCR2/3 nM mMIP-2, <<3 nM/0.1 to 60 nM hCXCL8/human; % inhibition/[Sch527123]/ligand/CXCR1 species = 35%/300 nM/3 nM hCXCL8/ human & 50%/9.667 µM/3 nM hCXCL8/monkey) without affecting C5a- or fMLP-induced human neutrophil chemotaxis. Oral administration is shown to effectively reduce pulmonary neutrophilia in vivo among LPS- or V2O5-treated mice & rats (ED50 1.3 to 1.8 mg/kg), as well as among Cynomolgus monkeys receiving repeat bronchoscopy & lung lavage (ED50 0.3 mg/kg). Also reported to inhibit hCXCL8-dependent human melanoma A375SM chemotaxis & invasion (79% & 69% inhibition, respectively, with 10 ng/mL = 25 nM Sch527123; overnight at 37 °C;10 ng/mL hCXCL8) and effectively suppress A375SM proliferation both in cultures in vitro (31% inhibition by MTT assay post 72 h treatment with 1 µg/mL = 2.5 µM Sch527123) and in mice in vivo (79% inhibition on D50 post cancer cells injection; 100 mg/kg/d p.o. D1 through D22) with concomitant increased apoptotic cells (203% of control) and decreased microvessel density in tumor tissues (55% of control).
A cell-permeable, orally active, non-toxic, cyclobutenedione compound with anti-inflammatory properties. Acts as a specific, high-affinity, and potent allosteric antagonist of CXCR2 (IC50 = 2.6 nM for human; Kd = 49, 200, and 80 pM for human, rat, and cynomolgus, respectively). Also exhibits high potency against CXCR1 (IC50 = 36 nM for human, Kd = 3.9 and 41 nM for human and cynomolgus, respectively). Displays excellent selectivity over CXCR3, CCR5, and a large panel of GPCRs, enzymes and ion channels (~ 10 µM). Potently inhibits CXCL1- and CXCL8-induced chemotaxis in human neutrophils (hPMN; IC50<1 nM and 16 nM, respectively). Shown to suppress pulmonary neutrophilia (ED50 = 1.3 mg/kg), goblet cell hyperplasia (ED50 = 700 mg/kg), and increase bronchoalveolar lavage (BAL) mucin content (ED50 ≤ 1 mg/kg) in rats subjected to vanadium pentoxide induced pulmonary inflammation. Inhibits melanoma cell proliferation (~ 1 mg/ml) by suppressing the phosphorylation of ERK1/2. Diminishes the invasive potential of melanoma cells and blocks angiogenesis in mice (100 mg/kg, p.o., q.d for 21 days). Exhibits desirable pharmacokinetic properties.
Please note that the molecular weight for this compound is batch-specific due to variable water content.
Please note that the molecular weight for this compound is batch-specific due to variable water content.
生物化学的/生理学的作用
Cell permeable: yes
Primary Target
CXCR2
CXCR2
Reversible: yes
Secondary Target
CXCR1
CXCR1
包装
Packaged under inert gas
警告
Toxicity: Standard Handling (A)
再構成
Following reconstitution, aliquot and freeze (-20°C). Stock solutions are stable for up to 6 months at -20°C.
Use only fresh DMSO for reconstitution.
その他情報
Seiberling, M., et al. 2013. Int. Immunopharmacol.17, 178.
Salchow, K., et al. 2010. Br. J. Pharmacol.159, 1429.
Singh, S., et al. 2009. Clin. Cancer Res.15, 2380.
Chapman, R.W., et al. 2007. J. Pharmacol. Exp. Ther.322, 486.
Gonsiorek, W., et al. 2007. J. Pharmacol. Exp. Ther.322, 477.
Dwyer, M.P., et al. 2006. J. Med. Chem.49, 7603.
Salchow, K., et al. 2010. Br. J. Pharmacol.159, 1429.
Singh, S., et al. 2009. Clin. Cancer Res.15, 2380.
Chapman, R.W., et al. 2007. J. Pharmacol. Exp. Ther.322, 486.
Gonsiorek, W., et al. 2007. J. Pharmacol. Exp. Ther.322, 477.
Dwyer, M.P., et al. 2006. J. Med. Chem.49, 7603.
法的情報
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)