おすすめの製品
詳細
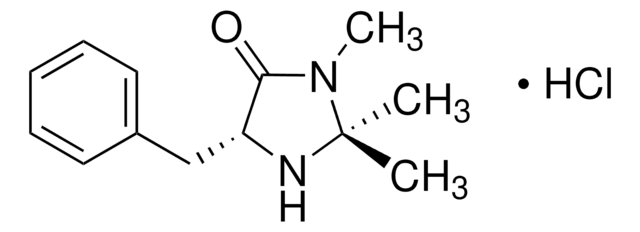
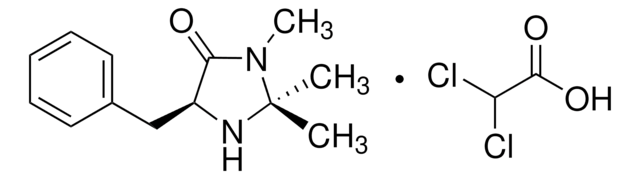
(2S,5S)-(-)-2-tert-Butyl-3-methyl-5-benzyl-4-imidazolidinone is a chiral imidazolidinone organocatalyst, developed by MacMillan and co-workers.
アプリケーション
(2S,5S)-(-)-2-tert-Butyl-3-methyl-5-benzyl-4-imidazolidinone is a second-generation MacMillan catalyst, which can be used as a chiral organocatalyst in:
- The chiral transformation reaction, including Friedel-Crafts and Mukaiyama-Michael reactions.
- The preparation of substituted spiroundecenetriones via asymmetric domino Knoevenagel/Diels-Alder reactions.
- The asymmetric synthesis of β-hydroxy aldehydes and their dimethylacetals via aldehyde-aldehyde aldol condensation reaction.
- The enantioselective α-fluorination of aldehydes using N-fluorobenzenesulfonamide as a fluorinating agent.
- The stereoselective preparation of (oxomethyl)oxabicyclo[3.2.1]octenones and tricyclic pyrroles via [4+3] cycloaddition of (trialkylsiloxy)pentadienals to furans.
Metal-free OrganoCatalyst technology for asymmetric catalysis. Catalyzes asymmetric indole alkylations, Friedel-Crafts alkylations, and a broad range of conjugate addition reactions in high enantiomeric excess.
特徴および利点
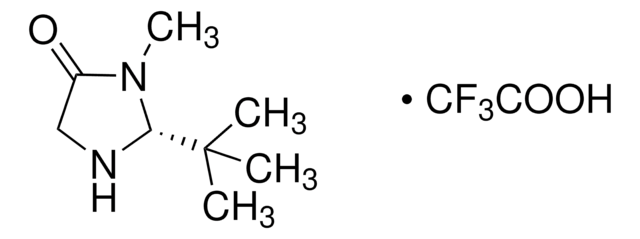
Advantages of MacMillan imidazolidinone organocatalysts:
- Superior enantiocontrol in numerous transformations
- High activities at low catalyst loadings
- Extraordinary functional group tolerance
法的情報
米国特許番号第6,369,243号および関連特許が適用されます。試験研究用用途にのみお使いください。
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
個人用保護具 (PPE)
Eyeshields, Gloves, type N95 (US)
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
663107-BULK:
663107-VAR:
663107-500MG:
663107-1G:
Asymmetric organocatalysis of 4+ 3 cycloaddition reactions
Harmata M, et al.
Journal of the American Chemical Society, 125(8), 2058-2059 (2003)
Evaluation of protonation sites in two MacMillan catalysts in solution by gas phase predissociation spectroscopy and electronic structure calculations
Tavares LC, et al.
Organic Chemistry, 134-145 (2020)
Teresa D Beeson et al.
Science (New York, N.Y.), 316(5824), 582-585 (2007-03-31)
The asymmetric alpha-addition of relatively nonpolar hydrocarbon substrates, such as allyl and aryl groups, to aldehydes and ketones remains a largely unsolved problem in organic synthesis, despite the wide potential utility of direct routes to such products. We reasoned that
The Importance of Iminium Geometry Control in Enamine Catalysis: Identification of a New Catalyst Architecture for Aldehyde-Aldehyde Couplings
Mangion IK, et al.
Angewandte Chemie (International Edition in English), 116(48), 6890-6892 (2004)
Nick A Paras et al.
Journal of the American Chemical Society, 124(27), 7894-7895 (2002-07-04)
The first enantioselective organocatalytic alkylation of electron-rich benzene rings with alpha,beta-unsaturated aldehydes has been accomplished. The use of iminium catalysis has provided a new strategy for the enantioselective construction of benzylic stereogenicity, an important chiral synthon for natural product and
資料
Discover Professor David MacMillan's groundbreaking metal-free asymmetric catalysis using imidazolidinone-based organocatalysts for versatile transformations.
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)
![(S)-α,α-ビス[3,5-ビス(トリフルオロメチル)フェニル]-2-ピロリジンメタノールトリメチルシリルエーテル 97%](/deepweb/assets/sigmaaldrich/product/structures/396/398/09a397b1-b5f5-420f-98da-adf9017cef56/640/09a397b1-b5f5-420f-98da-adf9017cef56.png)


