1A01250
USP
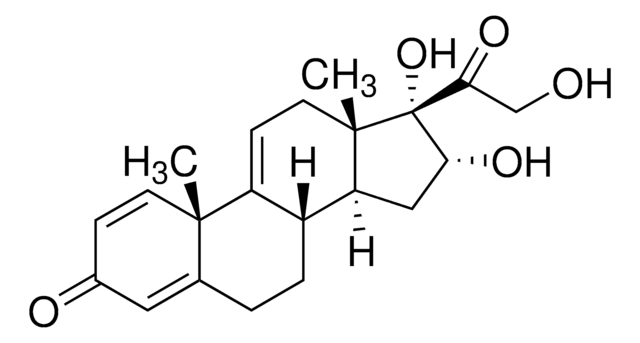
16ALPHA-HYDROXY-11-KETO PREDNISOLONE
Pharmaceutical Analytical Impurity (PAI)
Synonym(s):
(8S,9S,10R,13S,14S,16R,17S)-16,17-dihydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-7,8,9,10,12,13,14,15,16,17-decahydro-3H-cyclopenta[a]phenanthrene-3,11(6H)-dione
About This Item
Recommended Products
grade
pharmaceutical analytical impurity (PAI)
Agency
USP
API family
prednisolone
manufacturer/tradename
USP
application(s)
pharmaceutical
format
neat
storage temp.
2-8°C
General description
USP PAI are a product line of impurities suitable for research and analytical purposes, which help to ensure the quality and safety of medicines.
Associated Drug Substance: Budesonide
Therapeutic Area: Steroids
For more information about this PAI, visit here.
Application
Features and Benefits
1. Conduct analytical tests during early formulation feasibility studies.
2. Determine degradation impurities produced during stress studies.
3. Develop, validate, and transfer analytical methods.
4. Perform spiking studies during process R&D to demonstrate depletion upon recrystallization.
5. Record retention times and/or spectra
6. Determine relative response factors.
7. Identify unknown impurities that formed during ICH stability conditions.
8. Identify impurities that are present in the Reference Listed Drug
9. Test for and profile impurities not listed in drug substance and drug product monographs.
Analysis Note
Other Notes
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
JAN Code
1A01250-25MG:
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service