SBR00014
Daptomycin Ready Made Solution
1 mg/mL in DMSO
Synonym(s):
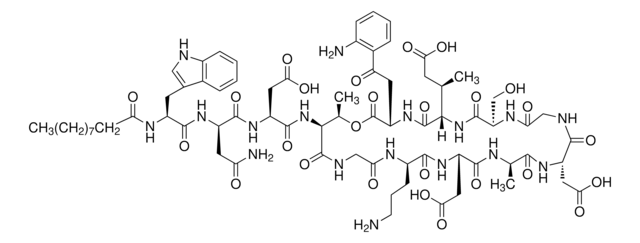
N-decanoyl-L-tryptophyl-L-asparaginyl-L-aspartyl-L-threonylglycyl-L-ornithyl-L-aspartyl-D-alanyl-L-aspartylglycyl-D-seryl-threo-3-methyl-L-glutamyl-3-anthraniloyl-L-alanine[egr]1-lactone
About This Item
Recommended Products
biological source
Streptomyces roseosporus
Quality Level
description
Ready Made Solution
form
liquid
storage condition
(Keep container tightly closed in a dry and well-ventilated place.)
concentration
1 mg/mL in DMSO
color
colorless to faint yellow
antibiotic activity spectrum
Gram-positive bacteria
Mode of action
DNA synthesis | interferes
protein synthesis | interferes
shipped in
dry ice
storage temp.
−20°C
SMILES string
O=C(NCC(N[C@H](CCCN)C(N[C@@H](CC(O)=O)C(N[C@H](C)C(N[C@@H](CC(O)=O)C(NC1)=O)=O)=O)=O)=O)[C@@H](NC([C@H](CC(O)=O)NC([C@@H](CC(N)=O)NC([C@H](CC2=CNC3=C2C=CC=C3)NC(CCCCCCCCC)=O)=O)=O)=O)[C@@H](C)OC([C@H](CC(C4=C(N)C=CC=C4)=O)NC([C@@]([C@H](C)CC(O)=O)([H])NC(
InChI
1S/C72H101N17O26/c1-5-6-7-8-9-10-11-22-53(93)81-44(25-38-31-76-42-20-15-13-17-39(38)42)66(108)84-45(27-52(75)92)67(109)86-48(30-59(102)103)68(110)89-61-37(4)115-72(114)49(26-51(91)40-18-12-14-19-41(40)74)87-71(113)60(35(2)24-56(96)97)88-69(111)50(34-90)82-55(95)32-77-63(105)46(28-57(98)99)83-62(104)36(3)79-65(107)47(29-58(100)101)85-64(106)43(21-16-23-73)80-54(94)33-78-70(61)112/h12-15,17-20,31,35-37,43-50,60-61,76,90H,5-11,16,21-30,32-34,73-74H2,1-4H3,(H2,75,92)(H,77,105)(H,78,112)(H,79,107)(H,80,94)(H,81,93)(H,82,95)(H,83,104)(H,84,108)(H,85,106)(H,86,109)(H,87,113)(H,88,111)(H,89,110)(H,96,97)(H,98,99)(H,100,101)(H,102,103)/t35?,36-,37?,43+,44+,45+,46+,47+,48+,49+,50-,60+,61?/m1/s1
InChI key
DOAKLVKFURWEDJ-OFNKPWESSA-N
Related Categories
Biochem/physiol Actions
The mode of action of daptomycin is quite different from that of any other antibiotic. Daptomycin binds to bacterial membranes and causes a rapid depolarization of membrane potential. This loss of membrane potential causes inhibition of protein, DNA, and RNA synthesis, which results in bacterial cell death.
Packaging
Preparation Note
Other Notes
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
188.6 °F
Flash Point(C)
87 °C
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
FSL
Group 4: Flammable liquids
Type 3 petroleums
Hazardous rank III
Water insoluble liquid
JAN Code
SBR00014-0.5ML:
SBR00014-0.5ML-PW:
SBR00014-BULK:
SBR00014-VAR:
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service