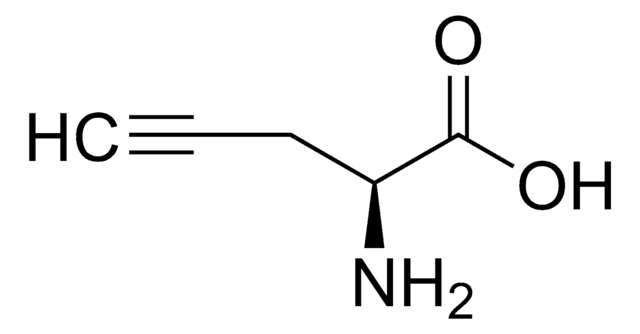
P7888
DL-Propargylglycine
cystathionine γ-lyase inhibitor
Synonym(s):
2-Amino-4-pentynoic acid, PAG
About This Item
Recommended Products
Assay
≥98% (TLC)
form
powder
color
white
application(s)
cell analysis
storage temp.
−20°C
SMILES string
NC(CC#C)C(O)=O
InChI
1S/C5H7NO2/c1-2-3-4(6)5(7)8/h1,4H,3,6H2,(H,7,8)
InChI key
DGYHPLMPMRKMPD-UHFFFAOYSA-N
Related Categories
Application
- Metabolic Labeling Strategy Boosted Antibacterial Efficiency for Photothermal and Photodynamic Synergistic Bacteria-Infected Wound Therapy.: This study demonstrates the enhanced antibacterial efficiency of a metabolic labeling strategy using ᴅʟ-Propargylglycine in photothermal and photodynamic therapy for treating bacteria-infected wounds (Li et al., 2022).
- Metabolic Labeling of Peptidoglycan with NIR-II Dye Enables In Vivo Imaging of Gut Microbiota.: This research utilizes ᴅʟ-Propargylglycine for metabolic labeling, allowing near-infrared II imaging of gut microbiota in vivo, providing new insights into microbiome studies (Wang et al., 2020).
- Hydrogen sulfide upregulated mRNA expressions of sodium bicarbonate cotransporter1, trefoil factor1 and trefoil factor2 in gastric mucosa in rats.: Investigates the role of ᴅʟ-Propargylglycine, in upregulating specific mRNA expressions in gastric mucosa, with implications for gastrointestinal research (Cheraghi et al., 2016).
Biochem/physiol Actions
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
JAN Code
P7888-100MG:
P7888-BULK:
P7888-250MG:
P7888-VAR:
P7888-5G:
P7888-1G:
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service