M0534
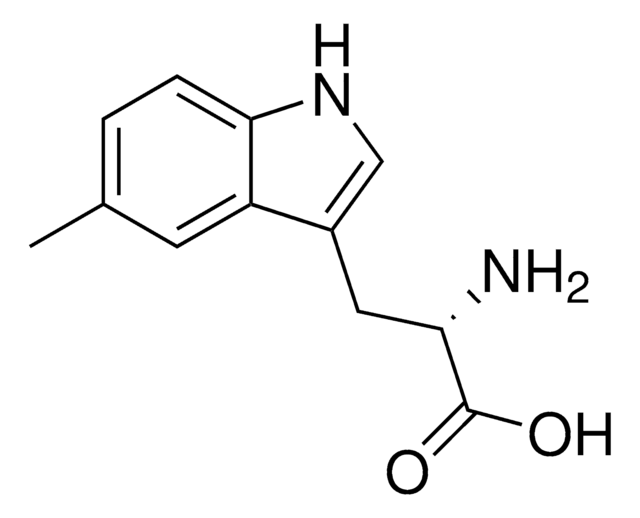
5-Methyl-DL-tryptophan
tryptophan analog
Synonym(s):
5-Methyltryptophan
About This Item
Recommended Products
Quality Level
Assay
≥97% (TLC)
form
powder
color
white to faint yellow
mp
280-282 °C
application(s)
peptide synthesis
storage temp.
2-8°C
SMILES string
Cc1ccc2[nH]cc(CC(N)C(O)=O)c2c1
InChI
1S/C12H14N2O2/c1-7-2-3-11-9(4-7)8(6-14-11)5-10(13)12(15)16/h2-4,6,10,14H,5,13H2,1H3,(H,15,16)
InChI key
HUNCSWANZMJLPM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Amelioration of colitis through modulation of gut microbiota: The metabolite 5-Methyl-ᴅʟ-tryptophan (5-MT), derived from Angelica sinensis polysaccharides, was found to ameliorate colitis by modulating gut microbiota and the TLR4/MyD88/NF-κB signaling pathway. This suggests a potential application of 5-MT in inflammatory bowel disease research and therapy (Zou et al., 2023).
- Enzymatic synthesis of tryptophan derivatives: A study on the one-pot enantioselective synthesis of (S)-spirobrassinin and non-natural (S)-methylspirobrassinin from amino acids highlights a method using a turnip enzyme. This research outlines a novel approach to synthesizing tryptophan derivatives, potentially useful in biochemical assays (Ryu et al., 2021).
Biochem/physiol Actions
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
JAN Code
M0534-BULK:
M0534-250MG-PW:
M0534-1G-PW:
M0534-100MG:
M0534-VAR:
M0534-250MG:
M0534-5G:
M0534-1G:
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service