Recommended Products
biological source
mouse
Quality Level
100
500
conjugate
unconjugated
antibody form
culture supernatant
antibody product type
primary antibodies
clone
PWS44, monoclonal
description
For In Vitro Diagnostic Use in Select Regions (See Chart)
form
buffered aqueous solution
species reactivity
human
packaging
vial of 0.1 mL concentrate (133M-14)
vial of 0.5 mL concentrate (133M-15)
bottle of 1.0 mL predilute (133M-17)
vial of 1.0 mL concentrate (133M-16)
bottle of 7.0 mL predilute (133M-18)
manufacturer/tradename
Cell Marque™
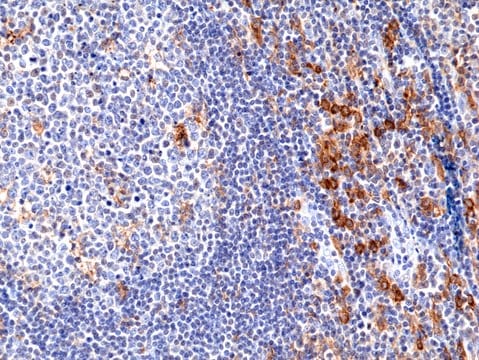
technique(s)
immunohistochemistry (formalin-fixed, paraffin-embedded sections): 1:20-1:80
isotype
IgG2b
control
acute myeloid leukemia with monocytic
shipped in
wet ice
storage temp.
2-8°C
visualization
membranous
Gene Information
human ... CD33(945)
Related Categories
General description
Quality
 IVD |  IVD |  IVD |  RUO |
Physical form
Preparation Note
Other Notes
Legal Information
Not finding the right product?
Try our Product Selector Tool.
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
JAN Code
133M-11:
133M-14:
133M-17-RUO:
133M-18:
133M-12:
133M-17:
133M-14-RUO:
133M-16:
133M-16-RUO:
133M-15:
133M-15-RUO:
133M-18-RUO:
133M-13:
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service