15256
BSA+TMCS
for GC derivatization, LiChropur™, 93.0-97.0% (GC)
Synonym(s):
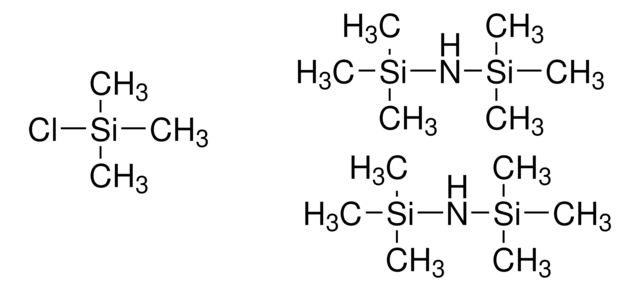
Bis(trimethylsilyl)acetamide + Trimethylchlorosilane
About This Item
Recommended Products
grade
for GC derivatization
Quality Level
Assay
93.0-97.0% (GC)
quality
LiChropur™
composition
trimethylchlorosilane (minor component), 3.0-5.0% GC
reaction suitability
reagent type: derivatization reagent
reaction type: Silylations
technique(s)
gas chromatography (GC): suitable
Related Categories
General description
Application
Features and Benefits
- BSA+TMCS has good solvent properties and can function as a silylation reagent without additional solvents.
- Alternatively, the mixture is very soluble in most commonly used silylation solvents.
- This combination is extremely sensitive to moisture and should be handled under dry conditions.
Other Notes
Legal Information
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Flam. Liq. 2 - Skin Corr. 1A
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
53.6 °F - closed cup
Flash Point(C)
12 °C - closed cup
Personal Protective Equipment
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
FSL
Group 4: Flammable liquids
Type 1 petroleums
Hazardous rank II
Water insoluble liquid
ISHL Indicated Name
Substances Subject to be Indicated Names
ISHL Notified Names
Substances Subject to be Notified Names
JAN Code
15256-VAR:
15256-50ML:
15256-10ML:
15256-BULK:
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Results of a study involving the ability few Fluka silylating reagents to form GC-MS-compatible trimethylsilylmethyl derivatives of NSAIDs
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service