441090
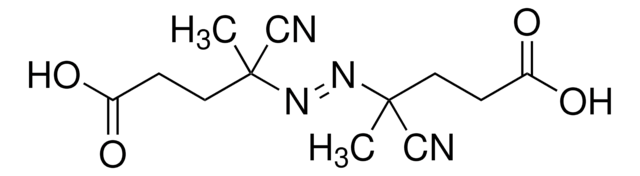
2,2′-Azobis(2-methylpropionitrile)
98%
Synonym(s):
α,α′-Azoisobutyronitrile, AIBN, Azobisisobutyronitrile, Free radical initiator
About This Item
Recommended Products
Assay
98%
form
powder
mp
102-104 °C (dec.) (lit.)
storage temp.
2-8°C
SMILES string
CC(C)(\N=N\C(C)(C)C#N)C#N
InChI
1S/C8H12N4/c1-7(2,5-9)11-12-8(3,4)6-10/h1-4H3/b12-11+
InChI key
OZAIFHULBGXAKX-VAWYXSNFSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Polystyrene by soap-free emulsion polymerization.
- Molecularly imprinted polymer(MIP) using 1-vinyl imidazole. MIP can be used to quantify acid violet 19 dye in river water samples.
Storage and Stability
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3 - Self-react. C
Supplementary Hazards
Storage Class Code
4.1A - Other explosive hazardous materials
WGK
WGK 2
Flash Point(F)
122.0 °F
Flash Point(C)
50 °C
Personal Protective Equipment
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
PDSCL
Deleterious substance
ISHL Indicated Name
Substances Subject to be Indicated Names
ISHL Notified Names
Substances Subject to be Notified Names
JAN Code
441090-100G:
441090-25G:
441090-BULK:
441090-VAR:
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
To keep pace with Moore′s Law, there is a continuing need in the semiconductor industry to achieve higher circuit density in microelectronic devices.
An article regarding common FAQs for initiators and stabilizers.
RAFT polymerization offers living characteristics to radical polymerization, contributing versatility to reversible deactivation radical polymerization methods.
Monomers for ophthalmic use aim for purity, reliability, and comfort, driving innovation for affordable contact lenses.
Protocols
RAFT polymerization offers precise control, enabling tailored synthesis of complex polymer structures.
We present an article about RAFT, or Reversible Addition/Fragmentation Chain Transfer, which is a form of living radical polymerization.
Polymerization via ATRP procedures demonstrated by Prof. Dave Haddleton's research group at the University of Warwick.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service