165336
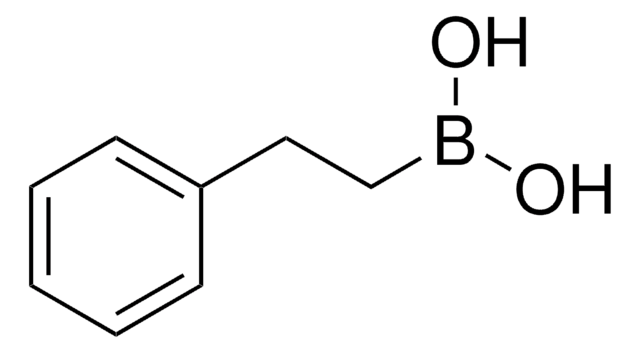
Methylboronic acid
97%
Synonym(s):
Methaneboronic acid
About This Item
Recommended Products
Assay
97%
form
solid
mp
91-94 °C (lit.)
SMILES string
CB(O)O
InChI
1S/CH5BO2/c1-2(3)4/h3-4H,1H3
InChI key
KTMKRRPZPWUYKK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- In the palladium-catalyzed Stille and Suzuki-Miyaura cross-couplings.
- In the microwave-heated heterogeneous palladium (Pd)-catalytized reactions.
- In ruthenium (Ru)-catalyzed silylation reactions
- To prepare bis(aminotropone) titanium (Ti) catalysts for ethylene polymerizations.
- In the enantioselective asymmetric bromoaminocyclization and bromoaminocyclization using amino-thiocarbamate catalysts.
- To prepare common building blocks for pharmaceuticals and agrochemicals.
- To prepare chrysin analogs by Suzuki-Miyaura coupling reactions.
- To prepare casein kinase I inhibitors.
- In the divergent C-H functionalizations directed by sulfonamide pharmacophores in drug discovery.
- In the synthesis of unsymmetrical monosulfides from disulfides via copper-catalyzed coupling with boronic acids.
- In a palladium-catalyzed coupling with enol tosylates.
- For derivatizing many carbohydrates and biologically active compounds for GLC analysis.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
JAN Code
165336-1G:
165336-VAR:
165336-BULK:
165336-5G:
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)






![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)