559398
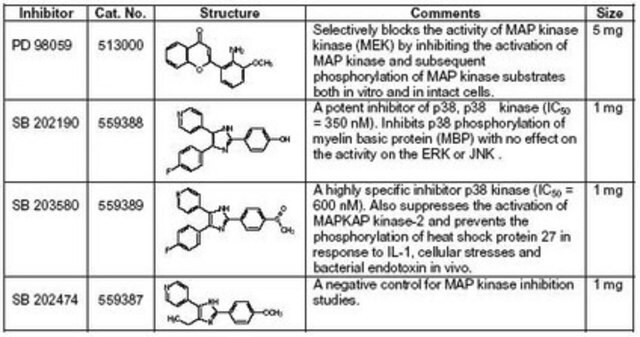
SB 203580
InSolution, ≥98%, 1 mg/ml, reversible inhibitor of p38 MAP kinase
Synonym(s):
InSolution SB 203580, InSolution p38 MAP Kinase Inhibitor XVI
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C21H16FN3OS
Molecular Weight:
377.43
UNSPSC Code:
12352200
NACRES:
NA.77
Recommended Products
Quality Level
Assay
≥98% (HPLC)
form
liquid
manufacturer/tradename
Calbiochem®
storage condition
OK to freeze
protect from light
shipped in
wet ice
storage temp.
−20°C
General description
A highly specific, cell-permeable inhibitor of p38 kinase (IC50 = 34 nM in vitro, 600 nM in cells). Also known as reactivating kinase (RK) and CSBP (cytokine synthesis anti-inflammatory drug binding protein). Does not significantly inhibit JNK or p42 MAP kinase even at 100 µM. Inhibits IL-1 and TNF-α production from LPS-stimulated human monocytes and the human monocyte cell line THP-1 (IC50 = 50-100 nM). SB 203580 has also been shown to be an effective inhibitor of inflammatory cytokine production in vivo in both mice and rats.
Biochem/physiol Actions
Cell permeable: yes
Product competes with ATP.
Reversible: yes
Target IC50: 34 nM in vitro, 600 nM in cells against p38 MAPK; 50-100 nM Inhibiting IL-1 and TNF-α production from LPS-stimulated human monocytes and the human monocyte cell line THP-1; 0.2-1.0 µM inhibiting platelet aggregation by collagen
Packaging
Packaged under inert gas
Warning
Toxicity: Irritant (B)
Physical form
A 1 mg/ml solution of SB 203580 (Cat. No. 559389) in anhydrous DMSO.
Other Notes
Powell, D.J., et al. 2003. Mol. Cell Biol.23, 7794.
Davies, S.P., et al. 2000. Biochem. J.351, 95.
Iwasaki, S., et al. 1999. J. Biol. Chem.274, 26503.
Gallagher, T.F., et al. 1997. Bioorg. Med. Chem. 5, 49.
LoGrasso, P.V., et al. 1997. Biochem. 36, 10422.
Hazzalin, C.A., et al. 1996. Curr. Biol.6, 1028.
Kramer, R.M., et al. 1996. J. Biol. Chem.271, 27723.
Saklatvala, J., et al. 1996. J. Biol. Chem. 271, 6586.
Cuenda, A., et al. 1995. FEBS Lett.364, 229.
Gallagher, T.F., et al. 1995. Bioorg. Med. Chem. Lett. 5, 1171.
Lee, J.C., et al. 1994. Nature 372, 739.
Davies, S.P., et al. 2000. Biochem. J.351, 95.
Iwasaki, S., et al. 1999. J. Biol. Chem.274, 26503.
Gallagher, T.F., et al. 1997. Bioorg. Med. Chem. 5, 49.
LoGrasso, P.V., et al. 1997. Biochem. 36, 10422.
Hazzalin, C.A., et al. 1996. Curr. Biol.6, 1028.
Kramer, R.M., et al. 1996. J. Biol. Chem.271, 27723.
Saklatvala, J., et al. 1996. J. Biol. Chem. 271, 6586.
Cuenda, A., et al. 1995. FEBS Lett.364, 229.
Gallagher, T.F., et al. 1995. Bioorg. Med. Chem. Lett. 5, 1171.
Lee, J.C., et al. 1994. Nature 372, 739.
Legal Information
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
188.6 °F - closed cup - (Dimethylsulfoxide)
Flash Point(C)
87 °C - closed cup - (Dimethylsulfoxide)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Kazunori Hamamura et al.
Cell biology international, 34(10), 1005-1012 (2010-05-29)
Osteoblast cells synthesize collagen-rich ECM (extracellular matrix) in response to various environmental cues, but little is known about ECM-dependent variations in phosphorylation patterns. Using MC3T3 E1 osteoblast-like cells and mouse whole-genome microarrays, we investigated molecular signalling affected by collagen-based ECMs.
Helen Willcockson et al.
Osteoarthritis and cartilage open, 3(1), 100136-100136 (2021-01-06)
In previous studies, we determined an association between increased serum and articular cartilage levels of CCL2 with osteoarthritis (OA) progression, cartilage damage and increased MMP13 in cartilage. Here we analyzed CCL2 downstream signaling mediators that lead to gene expression of
Huijeong Ahn et al.
Animal cells and systems, 28(1), 161-170 (2024-04-30)
Sonic vibration (SV), or vibroacoustic therapy, is applied to enhance local and systemic blood circulation and alleviate pain using low-frequency sine wave vibrations. However, there is limited scientific data on the mechanisms through which the benefits are achieved. In this
Ujunwa Cynthia Okoye-Okafor et al.
The Journal of experimental medicine, 219(11) (2022-09-03)
Thrombocytopenia, prevalent in the majority of patients with myeloid malignancies, such as myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML), is an independent adverse prognostic factor. Azacitidine (AZA), a mainstay therapeutic agent for stem cell transplant-ineligible patients with MDS/AML, often
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service