434191
Vinyl bromide solution
1.0 M in THF
Synonym(s):
Bromoethylene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
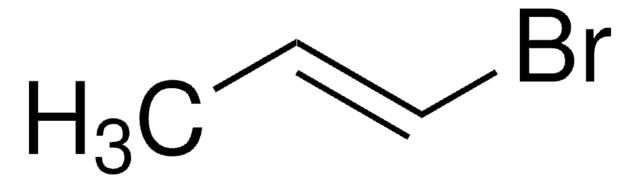
CH2=CHBr
CAS Number:
Molecular Weight:
106.95
Beilstein:
1361370
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
vapor pressure
12.46 psi ( 55 °C)
3.6 psi ( 20 °C)
Quality Level
form
liquid
concentration
1.0 M in THF
density
0.927 g/mL at 25 °C
storage temp.
2-8°C
SMILES string
BrC=C
InChI
1S/C2H3Br/c1-2-3/h2H,1H2
InChI key
INLLPKCGLOXCIV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Vinyl bromide solution belongs to haloalkenes andis highly reactive due to the presence of an unsaturated vinyl group. It can provide flame retardancy to polymers or materials when incorporated into their structure. It is also a versatile building block for polymerization, addition reactions, substitution reactions, and cross-coupling reactions like Suzuki-Miyaura and Negishi reactions. It can be used to introduce radiolabel into molecules for medical imaging.
Application
- Sequential Vinyl Radical Cyclization/Fixation of Carbon Dioxide through Electrochemical Reduction of Vinyl Bromide in the Presence of an Electron-Transfer Mediator: This study explores the electrochemical reduction of vinyl bromide with a focus on vinyl radical cyclization and carbon dioxide fixation (A Katayama, H Senboku, 2016).
- A Comparison of the Wavelength-Dependent Photochemical Reactions of Ozone with Vinyl Bromide and Fluoride in Argon Matrices: The study compares the photochemical reactions of vinyl bromide and fluoride with ozone, examining their behavior in argon matrices (BS Ault, 2021).
Vinyl bromide solution can be used as a precursor for stereoselective synthesis of chiral 2-vinyl-tetrahydronaphthalens via asymmetric reductive coupling. These chiral compounds are valuable building blocks for natural products, agrochemicals, and liquid crystals.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Carc. 1B - Eye Irrit. 2 - Flam. Liq. 2 - STOT SE 3
Target Organs
Central nervous system, Respiratory system
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
1.4 °F - closed cup
Flash Point(C)
-17 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Nickel/Copper Co-catalyzed Enantioselective Reductive Coupling of Oxabenzonorbornadienes with Vinyl Bromides
Yao Deng, et al.
advanced synthesis and catalysis, 365, 3265-3270 (2023)
Vinyl halides (selected): vinyl bromide.
Report on carcinogens : carcinogen profiles, 12, 437-438 (2011-08-25)
Derek R Boyd et al.
Organic & biomolecular chemistry, 5(3), 514-522 (2007-01-26)
Enantiopure trans-dihydrodiols have been obtained by a chemoenzymatic synthesis from the corresponding cis-dihydrodiol metabolites, obtained by dioxygenase-catalysed arene cis-dihydroxylation at the 2,3-bond of monosubstituted benzene substrates. This generally applicable, seven-step synthetic route to trans-dihydrodiols involves a regioselective hydrogenation and a
Ian Paterson et al.
Organic letters, 12(16), 3724-3727 (2010-08-14)
Using a combination of asymmetric vinylogous Mukaiyama aldol and Stille cross-coupling reactions, an advanced polyene fragment of the chivosazoles was prepared in a highly stereocontrolled manner. This key C1-C13 pentaene subunit, featuring the conjugated (2E,4Z,6E,8Z)-tetraenoate motif and anti-configured C10 and
Luo Yang et al.
Organic letters, 9(7), 1387-1390 (2007-03-06)
[structure: see text]. Catalyzed by CuI/N,N-dimethylglycine, oxiranecarboxamides underwent a highly efficient and stereoselective N-vinylation reaction with (Z)-1-aryl-2-bromoethenes to afford the corresponding enamides. The method has been applied to a straightforward synthesis of (-)-(2R,3S)-SB204900, the enantiomer of the natural product. Following
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service