325104
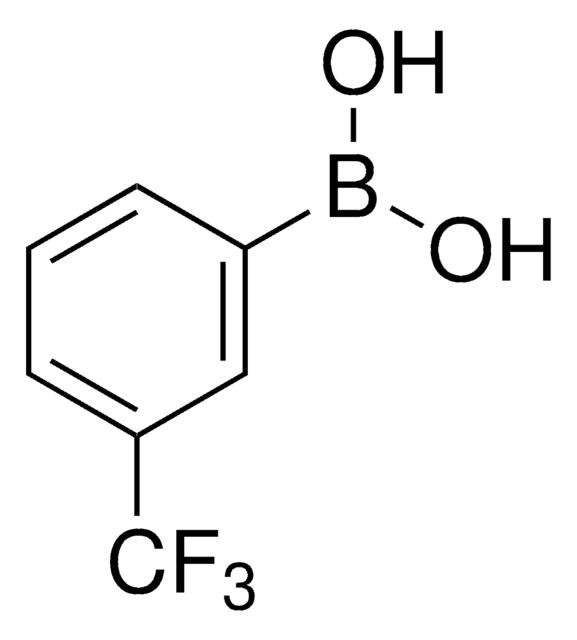
3-Nitrophenylboronic acid
≥97%
Synonym(s):
3-Nitrobenzeneboronic acid, m-Nitrobenzeneboronic acid, m-Nitrophenylboronic acid, NSC 401539, NSC 59739
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
O2NC6H4B(OH)2
CAS Number:
Molecular Weight:
166.93
Beilstein:
2938638
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥97%
form
powder
mp
284-285 °C (dec.) (lit.)
functional group
nitro
SMILES string
OB(O)c1cccc(c1)[N+]([O-])=O
InChI
1S/C6H6BNO4/c9-7(10)5-2-1-3-6(4-5)8(11)12/h1-4,9-10H
InChI key
ZNRGSYUVFVNSAW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Reactant involved in:
Additionally used as a reactant for synthesizing biologically active molecules such as:
- Copper-catalyzed arylation
- Palladium-catalyzed decarboxylative coupling
- Suzuki-Miyaura cross-coupling
- Oxidative carbocyclization / arylation
- Addition to arylpropargyl alcohols
Additionally used as a reactant for synthesizing biologically active molecules such as:
- Inhibitors of angiogenesis
- Biaryl-olefins with antiproliferative activities
Catalyzes ene carbocyclization of acetylenic dicarbonyl compounds
Other Notes
Contains varying amounts of anhydride
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
B J Johnson
Biochemistry, 20(21), 6103-6108 (1981-10-13)
Highly purified isoaccepting species of transfer ribonucleic acid (tRNA) were prepared by use of a polyacrylamide substituted with nitrobenzeneboronic acid functional groups. This method exploits the well-known ability of boronic acids to complex with RNA cis-diols. tRNA isoacceptors were obtained
K C Usher et al.
Biochemistry, 37(46), 16082-16092 (1998-11-18)
The structures of AmpC beta-lactamase from Escherichia coli, alone and in complex with a transition-state analogue, have been determined by X-ray crystallography. The native enzyme was determined to 2.0 A resolution, and the structure with the transition-state analogue m-aminophenylboronic acid
Fan Fei et al.
Analytical and bioanalytical chemistry, 398(3), 1349-1356 (2010-07-29)
The design of boronic acid sensors for photometric detection of carbohydrates has relied on exploiting differences in the thermodynamic stability of complex formation for molecular recognition. Herein, we introduce a direct method for analysis of sugar alcohols using 3-nitrophenylboronic acid
Aminophenyl- and nitrophenyl-labeled nucleoside triphosphates: synthesis, enzymatic incorporation, and electrochemical detection.
Hana Cahová et al.
Angewandte Chemie (International ed. in English), 47(11), 2059-2062 (2008-02-09)
Malcolm P Nicholls et al.
Organic & biomolecular chemistry, 2(10), 1434-1441 (2004-05-12)
The structures of thermodynamically stable aromatic boronic acid : cyclic carbohydrate chelates in aqueous alkaline media have been studied using 1H NMR spectroscopy and molecular modelling. It is found that interacting saccharides must necessarily possess a synperiplanar diol functionality for
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service