128279
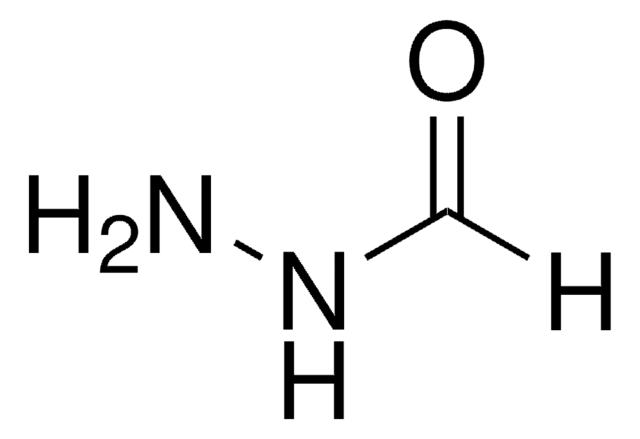
Ethyl hydrazinoacetate hydrochloride
97%
Synonym(s):
(Carbethoxymethyl)hydrazine hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
H2NNHCH2CO2C2H5 · HCl
CAS Number:
Molecular Weight:
154.60
Beilstein:
3912112
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
powder
mp
152-154 °C (lit.)
functional group
amine
ester
hydrazine
SMILES string
Cl[H].CCOC(=O)CNN
InChI
1S/C4H10N2O2.ClH/c1-2-8-4(7)3-6-5;/h6H,2-3,5H2,1H3;1H
InChI key
HZZRIIPYFPIKHR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Ethyl hydrazinoacetate hydrochloride has been used in the preparation of three novel copper(II) complexes as condensation derivatives of 2-pyridinecarboxaldehyde, 2-acetylpyridine and 2-quinolinecarboxaldehyde.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Copper (II) complexes of N heteroaromatic hydrazones: Synthesis, X-ray structure, magnetic behavior, and antibacterial activity.
Inorgorganica Chimica Acta, 362(6), 1996-2000 (2009)
Nenad Filipović et al.
Chemical biology & drug design, 84(3), 333-341 (2014-03-19)
Novel Pd(II) complex with N-heteroaromatic Schiff base ligand, derived from 8-quinolinecarboxaldehyde (q8a) and ethyl hydrazinoacetate (haOEt), was synthesized and characterized by analytical and spectroscopy methods. The structure of novel complex, as well as structures of its quinoline and pyridine analogues
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service