86843
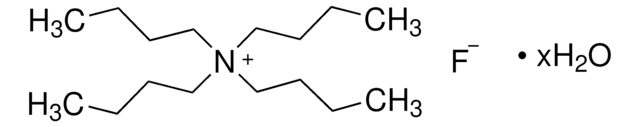
Tetrabutylammonium fluoride trihydrate
technical, ≥90% (T)
Synonym(s):
TBAF
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
[CH3(CH2)3]4NF · 3H2O
CAS Number:
Molecular Weight:
315.51
Beilstein:
3761900
EC Number:
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical
Quality Level
Assay
≥90% (T)
form
crystals
mp
62-63 °C (lit.)
SMILES string
O.O.O.[F-].CCCC[N+](CCCC)(CCCC)CCCC
InChI
1S/C16H36N.FH.3H2O/c1-5-9-13-17(14-10-6-2,15-11-7-3)16-12-8-4;;;;/h5-16H2,1-4H3;1H;3*1H2/q+1;;;;/p-1
InChI key
VEPTXBCIDSFGBF-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Application
Reactant for:
Preparation of deprotecting agents in preparation of cellulose derivatives
Synthesis of lipophilic peptides for DNA transfections in vivo
Dehydrobromination reactions
Preparation of deprotecting agents in preparation of cellulose derivatives
Synthesis of lipophilic peptides for DNA transfections in vivo
Dehydrobromination reactions
Tetrabutylammonium fluoride trihydrate may be used in the following studies:
- Synthesis of novel 3-O-(2-methoxyethyl)cellulose.
- To compose the novel solvent dimethyl sulfoxide (DMSO) /tetrabutylammonium fluoride trihydrate, used for the acetylation of linters cellulose.
- Synthesis of neutral organometallic fluoro complex.
- Synthesis of fluoroaromatic compounds.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Irrit. 2 - Repr. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Fluorodenitrations using tetrabutylammonium fluoride.
Clark JH and Smith DK.
Tetrahedron Letters, 26(18), 2233-2236 (1985)
Beatriz A P Ass et al.
Macromolecular bioscience, 4(11), 1008-1013 (2004-11-06)
The novel solvent dimethyl sulfoxide (DMSO)/tetrabutylammonium fluoride trihydrate (TBAF . 3H(2)O) was studied for acetylation of linters cellulose. In order to control the degree of substitution (DS), acetylation of the macromolecule was carried out at different reaction time and temperature
Effective preparation of cellulose derivatives in a new simple cellulose solvent.
Heinze T, et al.
Macromolecular Chemistry and Physics, 201(6), 627-631 (2000)
New solvents for cellulose: dimethyl sulfoxide/ammonium fluorides.
Kohler S and Heinze T.
Macromolecular Bioscience, 7(3), 307-314 (2007)
Tetrabutylammonium Fluoride.
Li HY, et al.
e-EROS Encyclopedia of Reagents for Organic Synthesis. (2007)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service