All Photos(1)
About This Item
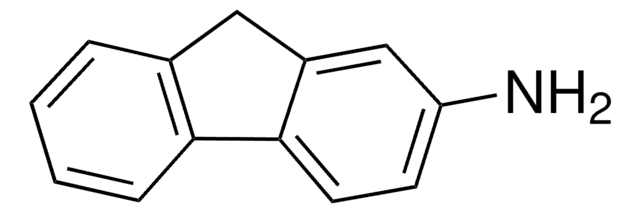
Empirical Formula (Hill Notation):
C18H13N
CAS Number:
Molecular Weight:
243.30
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
solid
mp
209-211 °C (lit.)
SMILES string
Nc1cc2c3ccccc3ccc2c4ccccc14
InChI
1S/C18H13N/c19-18-11-17-13-6-2-1-5-12(13)9-10-15(17)14-7-3-4-8-16(14)18/h1-11H,19H2
InChI key
KIVUHCNVDWYUNP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Produces tumors in mice.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Determination of polycyclic aromatic amines in skin by liquid chromatography with electrochemical detection.
L J Felice et al.
Journal of chromatography, 354, 442-448 (1986-02-28)
B G Lake et al.
Toxicology and applied pharmacology, 138(2), 231-241 (1996-06-01)
Precision-cut liver slices were prepared from male Sprague-Dawley rats (pretreated with or without Aroclor 1254), male Dunkin-Hartley guinea pigs, male cynomolgus monkeys, and humans. Liver slices were cultured for 24 hr using a dynamic organ culture system in medium containing
H Yamazaki et al.
Carcinogenesis, 15(3), 465-470 (1994-03-01)
In order to address the hypothesis that 6-aminochrysene (6-AC) is converted to genotoxic products by cytochrome P450 enzymes via two activation pathways (N-hydroxylation and epoxidation), the activation of 6-AC and trans-1,2-dihydro-1,2-dihydroxy-6-aminochrysene (6-AC-diol) to genotoxic metabolites was examined in rat and
D Lautier et al.
The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society, 36(6), 685-691 (1988-06-01)
Previous reports on the inhibitory effect of 6-amino-chrysene (6AC) on benzo(a)pyrene (BP) metabolism using single living cells have suggested that aryl hydrocarbon hydroxylase (AHH) is not the only pathway for 6AC metabolism. We present here results demonstrating that direct glucuronidation
J M Salmon et al.
Cytometry, 9(1), 25-32 (1988-01-01)
The identification and quantification of fluorescent compounds in a complex fluorescence spectra are always difficult, especially in the case of low signal:noise ratio. We propose a computerised method that allows the resolution of low light level complex fluorescence spectra into
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service