All Photos(1)
About This Item
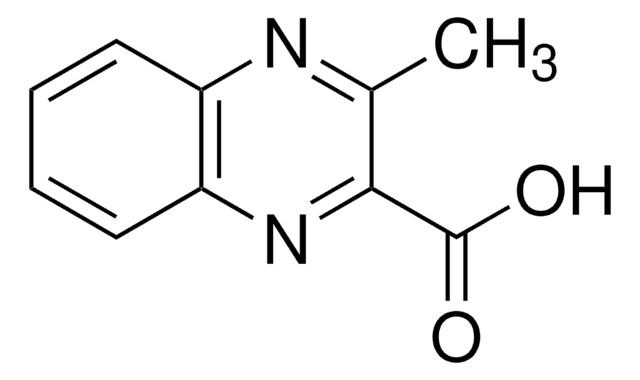
Empirical Formula (Hill Notation):
C9H6N2O2
CAS Number:
Molecular Weight:
174.16
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
208 °C (dec.) (lit.)
SMILES string
OC(=O)c1cnc2ccccc2n1
InChI
1S/C9H6N2O2/c12-9(13)8-5-10-6-3-1-2-4-7(6)11-8/h1-5H,(H,12,13)
InChI key
UPUZGXILYFKSGE-UHFFFAOYSA-N
General description
Linear and Freundlich adsorption isotherm coefficient of 2-quinoxalinecarboxylic acid has been evaluated.
Application
2-Quinoxalinecarboxylic acid has been used in the preparation of:
- N-(2-quinoxaloyl)-α-amino acids
- bisquinoxaloyl (bisquinoxalinecarbonyl) derivatives
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
M J Hutchinson et al.
The Analyst, 127(3), 342-346 (2002-05-09)
A method is described for the quantitative determination of quinoxaline-2-carboxylic acid (QCA), the marker residue for the veterinary drug carbadox, in swine liver. Tissue is subjected to alkaline hydrolysis followed by liquid-liquid extraction. QCA residues are cleaned up using automated
Zhen-Juan Duan et al.
Analytical and bioanalytical chemistry, 401(7), 2291-2299 (2011-08-23)
A new molecularly imprinted polymer (MIP), selective for major metabolites of quinoxaline-1,4-dioxides was firstly prepared by combining surface molecular imprinting technique with the sol-gel process. Methyl-3-quinoxaline-2-carboxylic acid (MQCA) was used as template, 3-aminopropyltriethoxysilane as functional monomer, and tetraethoxysilicane as cross-linker.
Quinoxaline studies. 13. N-(2-Quinoxaloyl)-alpha-amino acids.
S Gerchakov et al.
Journal of medicinal chemistry, 9(2), 266-268 (1966-03-01)
Dapeng Peng et al.
Food chemistry, 237, 290-296 (2017-08-03)
An immunoaffinity column (IAC) for the selective purification of 3-methyl-quinoxaline-2-carboxylic acid (MQCA) from porcine muscle and the liver as well as the methods for its determination by high-performance liquid chromatography with ultraviolet detection (HPLC-UV) and a colloidal gold-based immunochromatographic assay
Joshua A Hagen et al.
Sensors (Basel, Switzerland), 11(7), 6645-6655 (2011-12-14)
Zinc oxide field effect transistors (ZnO-FET), covalently functionalized with single stranded DNA aptamers, provide a highly selective platform for label-free small molecule sensing. The nanostructured surface morphology of ZnO provides high sensitivity and room temperature deposition allows for a wide
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service