All Photos(1)
About This Item
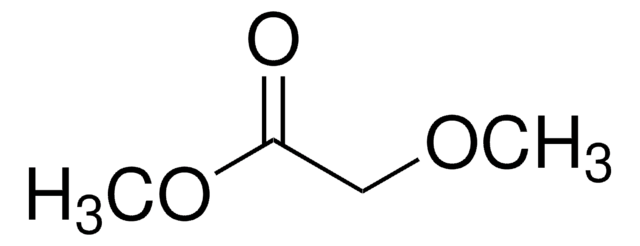
Linear Formula:
CH3OCH2COOC2H5
CAS Number:
Molecular Weight:
118.13
Beilstein:
1744761
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
liquid
refractive index
n20/D 1.401 (lit.)
bp
44-45.5 °C/9 mmHg (lit.)
density
1.007 g/mL at 25 °C (lit.)
SMILES string
CCOC(=O)COC
InChI
1S/C5H10O3/c1-3-8-5(6)4-7-2/h3-4H2,1-2H3
InChI key
JLEKJZUYWFJPMB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Ethyl methoxyacetate was used:
- as acyl donor during the preparation of enantiomers of several phenylethylamines
- as acylation reagent for the aminolysis of 1-phenylethanamine
- in an industrial, lipase-catalysed kinetic resolution of primary amine
Signal Word
Warning
Hazard Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
114.8 °F - closed cup
Flash Point(C)
46 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Ulf Hanefeld
Organic & biomolecular chemistry, 1(14), 2405-2415 (2003-09-06)
Commonly, hydrolase-catalysed reactions are used for kinetic resolutions of alcohols, amines and acids. Only a few applications in total syntheses have been described. Most of these examples are for the synthesis of amides and peptides. Here, the synthesis of all
Lourdes Muñoz et al.
Organic & biomolecular chemistry, 9(23), 8171-8177 (2011-10-06)
Both enantiomers of several phenylethylamines, structurally related to amphetamine, have been prepared in good yields and excellent enantiomeric purity by enzymatic kinetic resolution using CAL-B and ethyl methoxyacetate as the acyl donor. In the case of the 4-hydroxyderivative of amphetamine
A Basler
Mutation research, 174(1), 11-13 (1986-05-01)
Chinese hamsters were exposed to acetone, methyl ethyl ketone, ethyl acetate and 2-methoxy ethyl acetate, known to be strong inducers of aneuploidy in the yeast Saccharomyces cerevisiae. All solvents yielded negative results in the micronucleus test, whereas the vinca alkaloid
Saija Sirén et al.
Molecules (Basel, Switzerland), 25(4) (2020-02-23)
The enantiomers of aromatic 4-dibenzocyclooctynol (DIBO), used for radiolabeling and subsequent conjugation of biomolecules to form radioligands for positron emission tomography (PET), were separated by kinetic resolution using lipase A from Candida antarctica (CAL-A). In optimized conditions, (R)-DIBO [(R)-1, ee
C Saito et al.
Biotechnic & histochemistry : official publication of the Biological Stain Commission, 74(1), 40-48 (1999-04-06)
An in situ hybridization procedure resulting in both high resolution and sensitivity was established by using the removable methyl methacrylate resin, Technovit 9100. Young bicellular pollen of tobacco (Nicotiana tabacum L. SR-1) was embedded in Technovit 9100 resin and sectioned.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service