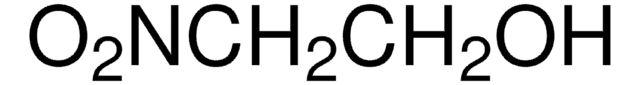192333
Ethyl nitroacetate
97%
Synonym(s):
2-Nitroacetic acid ethyl ester, Ethyl 2-nitroacetate, Nitroacetic acid ethyl ester
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
NO2CH2CO2C2H5
CAS Number:
Molecular Weight:
133.10
Beilstein:
1210027
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.424 (lit.)
bp
105-107 °C/25 mmHg (lit.)
density
1.199 g/mL at 25 °C (lit.)
functional group
amine
ester
nitro
SMILES string
CCOC(=O)C[N+]([O-])=O
InChI
1S/C4H7NO4/c1-2-9-4(6)3-5(7)8/h2-3H2,1H3
InChI key
FTKASJMIPSSXBP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Ethyl nitroacetate has been used in:
- synthesis of γ-oxoacids via Michael addition reaction with α,β-unsaturated ketones
- fuctionalization of C4-position on pyrimidine and C6-position on 2′-deoxyguanosine to produce novel nucleosides
- facile synthesis of α,α-diisobutylglycine
- synthesis of DL-4,4-difluoroglutamic acid
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
197.6 °F - closed cup
Flash Point(C)
92 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Victor Timoshchuk
Nucleosides, nucleotides & nucleic acids, 24(5-7), 1043-1046 (2005-10-27)
A study of C-nucleophilic substitution at the C4-position on pyrimidine and C6-position on 2'-deoxyguanosine to produce novel nucleosides is presented with the spectroscopic properties of their respective substitution products. C4-(1,2,4-triazol-1-yl) pyrimidine nucleosides 1 were treated with nitroalkanes, malononitrile, acetylacetone, ethyl
Elena Trogu et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 15(32), 7940-7948 (2009-04-24)
Ethyl nitroacetate (1) reacts with electron-poor olefins in the presence of a base to give either the Michael adducts 3 or the isoxazoline cycloadducts 4, resulting from water elimination. The proportions of the two products depend on the reaction conditions
Yanwen Fu et al.
The Journal of organic chemistry, 68(25), 9854-9857 (2003-12-06)
alpha,alpha-Diisobutylglycine has been synthesized using a Pd-mediated dialkylation of ethyl nitroacetate as a key first step. The free alphaalphaAA is N(alpha)-protected and has been applied to the assembly of conformationally constrained peptide analogues. Mixed anhydrides from BOP-Cl and Fmoc-alphaalphaAA-OH are
Indranil Bhattacharjee et al.
Physical chemistry chemical physics : PCCP, 20(9), 6060-6072 (2017-12-23)
Achieving synthetic control over light-driven molecular dynamics is essential for designing complex molecule-based devices. Here we design a novel coumarin-imidazole conjugate (1) whose excited state structural dynamics are primarily controlled by a distant intramolecular H-bonding interaction within the backbone. The
T Tsukamoto et al.
Journal of medicinal chemistry, 39(1), 66-72 (1996-01-05)
DL-4,4-Difluoroglutamic acid (DL-4,4-F2Glu) and its methotrexate analogue, DL-gamma,gamma-difluoromethotrexate (DL-gamma,gamma-F2MTX), were synthesized and evaluated as alternate substrates or inhibitors of folate-dependent enzymes. Synthesis of DL-4,4-F2Glu involved the nitroaldol reaction of ethyl nitroacetate with a difluorinated aldehyde ethyl hemiacetal as a key
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service