Improving Glycoform Coverage for Mucin Preparations
Mucins can be challenging to analyze properly when using traditional tryptic digestion due to their heavily glycosylated domains. Here we explore the use of the mucin-selective protease, Mucinase StcE, for tryptic-digest sample preparation of two clinically relevant mucin domain macromolecules (MUC16 and Podocalyxin) and assess its potential to improve the detection of glycoforms and glycopeptides.
Read more about
- What Is A Mucin?
- Mucins and Cancer
- Mucin Glycoprotein Digestion Methods
- Glycoproteomic Analysis
- Implications of Biomarker Testing in Mucosal Tissue Samples
- Related Products
- References
What Is A Mucin?
Proteins containing mucin domains have long-standing clinical relevance. Mucins are a family of large, densely O-glycosylated proteins that are found in and make up much of the mucus secretions and transmembrane glycoproteins of cell surfaces.1 Mucins function as a barrier for epithelial cells and are vital for the lubrication and protection of surfaces. Investigating the biological functions of mucins at the molecular scale is a challenge, as few tools are available to probe mucin domains. The dense glycosylation of these domains can shield probing using traditional techniques like tryptic digest.
Works by Malaker, et. al. show a mucin-selective protease (Mucinase StcE) that is useful for the analysis of heavily glycosylated mucin domains.2 Secreted protease of C1 esterase inhibitor (StcE) is a bacterial metalloprotease from Escherichia coli that can be used to digest densely O-glycosylated mucins.
Mucins and Cancer
Mucins are thought to play a role in some types of cancer and are even used as a diagnostic marker. MUC16 (length 14507 AA residues, Mass a 1.5MDa) is a large 3-5 million Da transmembrane mucin protein expressed in core glycans. It has densely O- and N-glycosylated domains which make it and other mucins resistant to digestion by proteases. N-linked glycosylation found on MUC16 is primarily high mannose and complex bisecting type N-linked glycans. It contains numerous disulfide bridges that cause the association of several secreted molecules, providing an extraordinarily large gel-like matrix in the extracellular space or the lumen of secretory ducts. CA125, a commonly used biomarker for lung and ovarian cancers, is a peptide epitope of MUC16.3 But its use as a definitive diagnostic marker suffers from insufficient specificity in immunoassays because while it is elevated in ovarian cancer it is present in natural abundance in normal tissues as well, making it difficult to accurately assess. Glycosylation can serve as a 2nd dimension to enhance its specificity as the glycoprofile of CA125 can vary in a disease-dependent manner.4
Podocalyxin (length 558 AA residues, Mass 59 kDa) is another common cancer biomarker.5 It is an anti-adhesive densely O-glycosylated transmembrane sialomucin that has been implicated in the development of more aggressive forms of breast and prostate cancer. It is present in a variety of tissues throughout the body that have tissue-specific glycosylation. This tissue-specific profile can be perturbed in cancer to the extent to which antibody interaction is impacted.6
StcE is expected to digest these glycoproteins into smaller chunks to expose their inner core which may then be accessible to sequencing grade proteases to enhance glycopeptide identification and N- and O-glycosylation characterization. Having tools to probe the glycan occupancy and glycoforms thoroughly are important for developments in glyco-oncology and glycosylation-dependent clinical applications.
Mucin Glycoprotein Digestion Methods
Target mucin proteins Podocalyxin (PODXL, 59 kDa) and MUC16 (recombinant Met13360-Gln14347, 170 – 200 kDa) (both from R&D Systems®) were put through standard tryptic digest preparations including reduction and alkylation with and without pre-treatment with Mucinase StcE. Figure 1 shows a schematic of the pre-treatment test process. Sequence coverage for glycopeptides and several identified glycoforms were then compared between the two conditions.
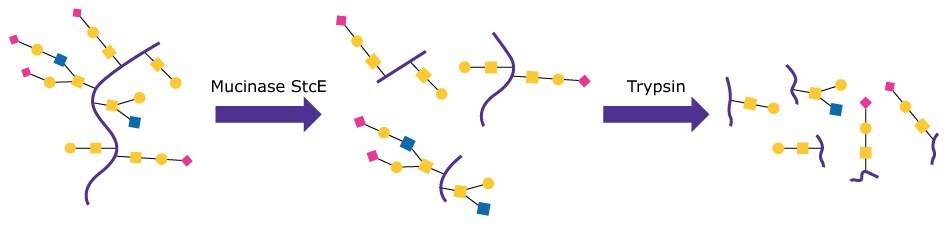
Figure 1. Schematic of the digestion series for Mucinase StcE/Trypsin treatment.
Mucinase StcE was prepared by reconstituting 100 μg of enzyme in PBS to a concentration of 1 μg/μL. Target proteins were prepared by reconstituting 50 μg of the target protein in PBS at 1 μg/μL. The Mucinase StcE digestion was done using a 1:10 E:S ratio. Specifically, 5 μL of target protein, 1.5 μL of 1% ProteaseMAX™ surfactant (from Promega™ Corporation) in 50 mM NH4HCO3, 6.5 μL of 50 mM NH4HCO3, and 2 μL of Mucinase StcE (test) or buffer (negative control) were combined and mixed, then incubated at 37 °C for 3 hrs. Samples were then taken through common reduction and alkylation methods using DTT and iodoacetamide before being treated with trypsin. Samples were desalted using SPE cleanup tips, dried, and reconstituted in 0.1% formic acid in water for LC/MS.
Samples were run on a nanoAcquity UPLC system coupled to the Orbitrap Fusion™ Lumos™ Tribrid™ mass spectrometer with ETD, an nLC-MS/MS system (from Thermo Scientific™). For enhanced DDA glycopeptide fragmentation, three stepped-collision energies were used, and data was acquired with both HCD and EThcD modes for every precursor selected. Raw data were processed using Byonic, MetaMorpheus, and BiopharmaFinder software packages to analyze N- and O-glycosylation, and glycoforms. Results were filtered for a maximum of 1% false discovery rate.
Glycoproteomic Analysis
Glycoproteomic analysis of Podocalyxin and MUC16 with and without Mucinase StcE pre-treatment was conducted through LC-MS and the number of MS peaks was counted (Figure 2).
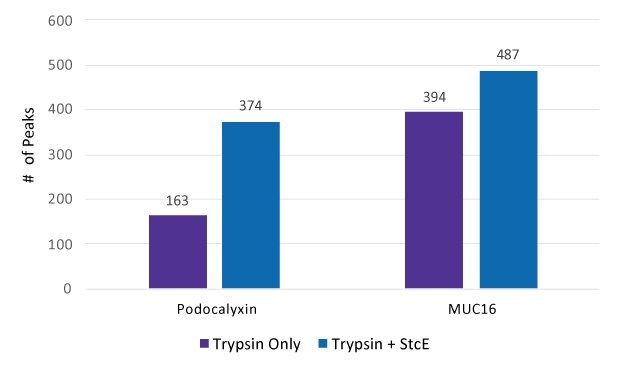
Figure 2.Count of peaks seen in spectra after treatment by Trypsin only and by Trypsin + Mucinase StcE.
The numbers of glycopeptides and glycoforms identified in Podocalyxin and MUC16 with or without Mucinase StcE digestion were determined (Figure 3). MUC16 samples treated with StcE resulted in 23 unique glycopeptides (12 N-linked, 11 O-linked) versus 14 unique glycopeptides (12 N-linked, 2 O-linked) in the untreated samples. Podocalyxin samples treated with StcE resulted in 23 unique glycopeptides (5 N-linked, 18 O-linked) versus 16 glycopeptides (4 N-linked, 12 O-linked) in the untreated samples. MUC16 samples treated with StcE resulted in 88 glycoforms (67 N-linked, 21 O-linked) versus 56 (53 N-linked, 3 O-linked) from untreated. Podocalyxin samples treated with StcE resulted in 260 glycoforms (61 N-linked, 199 O-linked) versus 193 (69 N-linked,124 O-linked) from untreated.

Figure 3. N-linked and O-linked glycopeptides and glycoforms identified after treatment of (A) MUC16 and (B) Podocalyxin with (blue) and without (purple) Mucinase StcE.
Implications of Biomarker Testing in Mucosal Tissue Samples
Digestion of mucin-type glycoproteins with Mucinase StcE prior to glycoproteomic analysis shows an increase in the number of glycopeptides identified and a very strong increase in the number of glycoforms identified. This is presumably because the smaller glycopeptides formed from the Mucinase StcE digestion are more accessible by trypsin and/or high-energy collision. While untested, this would likely apply to other enzymatic treatments including enzymatic glycan release and other specific endoproteases (e.g., EndoLysC, EndoArgC, etc.) useful for glycomics and proteomics work. These results suggest that the preparation of mucosal tissue samples used for biomarker testing may yield more information and increased signal if treated with Mucinase StcE prior to glycomics and proteomics workflows. It also provides a unique platform for rapid and comprehensive analysis of site-specific glycosylation in complex proteins or mixtures.
References
Per continuare a leggere, autenticati o crea un account.
Non hai un Account?