High-throughput qPCR using SYBR® Green is a demanding application that requires consistent and reproducible results from low-volume, high-speed assays.
The combination of the Eppendorf Mastercycler® ep realplex4 S real-time PCR instrument and KAPA SYBR® FAST qPCR Kits from Kapa Biosystems provides an industry-leading solution for high-performance, fast qPCR without compromising reaction efficiency and reproducibility.
Introduction
The number of applications utilizing real-time PCR (qPCR) is expanding rapidly. The improved accuracy of results relative to end-point PCR, multiplexing capability, reduced risk of amplicon contamination, and streamlined time-to-results from closed-tube qPCR assays are factors contributing to the growth of real-time PCR. Common qPCR applications include: gene expression profiling, genotyping, copy number detection, validation of siRNA knockdown, miRNA assays, bacterial and viral load determination, ChIP qPCR, and next-generation sequencing library quantification.
Standard SYBR® Green I-based qPCR cycling protocols take between 1 hr 40 min and 2 hr 8 min, depending on the ramp rate and data acquisition times of the specific qPCR instrument used (Figure 1). Faster cycling protocols (~45 min) that do not compromise reaction efficiency typically require instrumentation with increased thermal ramp rates, uniform heating across the block, and specially formulated reagents.
The Eppendorf realplex4 S real-time PCR system features a super-fast, Peltier-controlled thermoblock that heats up at 6ºC/sec and cools down at 4ºC/sec, which allows completion of a 40-cycle qPCR reaction in less than 1 hr. These Peltier units also ensure accurate temperature control, as well as high block homogeneity, which are essential to high-quality qPCR reactions. The optical module comprises an array of 96 LEDs that are pre-selected and equalized so that they excite all wells uniformly. This setup also eliminates the requirement for ROX reference dye that is used to normalize fluorescent signals by many conventional qPCR instruments. The realplex4 also utilizes the Photo Multiplier Tube (PMT) for signal detection, which is the most sensitive and affordable detection technique currently available.
Wild-type Taq DNA Polymerase is theoretically capable of a 30 sec combined annealing and extension time required for short qPCR amplicons (Lawyer 1993). However, inhibition due to SYBR® Green I dye, steric hindrance from templates with a high GC or AT content, and lower enzyme activity at 60ºC can result in compromised reaction efficiencies or even reaction failure when fast qPCR cycling protocols are used in conjunction with reagents containing wild-type Taq.
In contrast, the KAPA SYBR® FAST qPCR Kit contains a second-generation DNA polymerase with an intrinsic ability to synthesize DNA much faster than wild-type Taq. The KAPA SYBR® DNA Polymerase was engineered through a process of molecular evolution in the presence of elevated concentrations of SYBR® Green I dye, resulting in an enzyme that exhibits less SYBR® Green inhibition and a faster extension rate than wildtype Taq. High signal strength, which is particularly useful when performing low-volume qPCR, is achieved by an increased concentration of SYBR® Green I dye in KAPA SYBR® FAST master mix. KAPA SYBR® FAST qPCR Kits are supplied with an antibody-based hot start, enabling a short initial activation hold time at 95ºC. PCR protocols using KAPA SYBR® DNA Polymerase are primarily based on reduced extension times that allow for a significant reduction in PCR cycling time without the risk of compromising reaction performance.

Figure 1.Total qPCR times for either a standard reaction protocol (10 min at 95ºC activation, 40 cycles of 15 sec at 95 ºC and 60 sec at 60 ºC, 60 ºC – 95 ºC melt) or a fast protocol (3 min at 95ºC activation, 40 cycles of 3 sec at 95 ºC and 20 sec at 60 ºC, 60 ºC – 95 ºC melt). Reactions were performed on a slow ramp rate qPCR thermocycler (avg. ramp rate 1 ºC/sec) or the fast Eppendorf realplex4 S real-time PCR system (6 ºC/sec heating and 4 ºC/sec cooling).
Methods
To demonstrate the ability to perform fast qPCR cycling without compromising performance, a set of nine 2-fold serial dilutions of human genomic DNA (hgDNA) was amplified using either a standard or fast cycling protocol. Amplification of the 150 bp ss48422848 amplicon was performed in full-skirted Eppendorf Twin.tec plates with optical clear heat-sealing film using the Eppendorf realplex4 S real-time PCR system.
The resulting log amplification plots and standard curves for reactions performed using the standard protocol (Figure 2) and fast protocol (Figure 3) indicate that both the CT and efficiency of the qPCR reactions have not been compromised as a result of using fast cycling protocols relative to standard cycling protocols (for a description of each protocol see Figure 1). The fast protocol results in a time saving of 56 min relative to the slow protocol when performed on the Eppendorf realplex4 S real-time PCR system.
Reduced running costs, particularly in combination with increased throughput, are always desirable. To illustrate the ability to use reduced reagent volumes with comparable results, three different reaction volumes (5 μL, 10 μL and 20 μL) were compared using the fast protocol (Figure 4). All three reaction volumes resulted in highly consistent amplification across a set of seven 2-fold serial dilutions of hgDNA when cycled using the fast protocol. KAPA SYBR® FAST qPCR Kits are formulated with a high concentration of SYBR® Green I dye that is capable of delivering high levels of fluorescence even at extremely low reaction volume. In conjunction with the benefit of reduced evaporation during qPCR by using the Eppendorf realplex4 S real-time PCR system, comparable results were obtained using low-volume qPCR reactions.
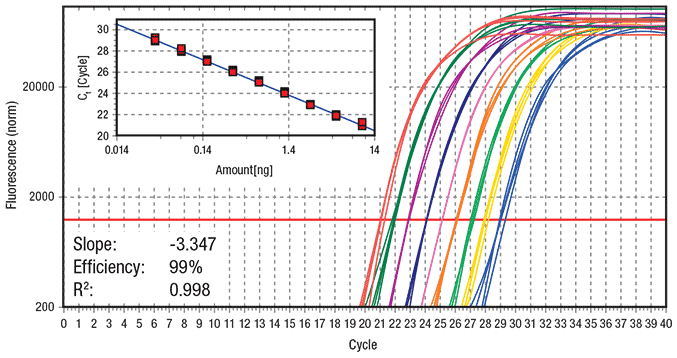
Figure 2.Standard cycling protocol (Run time = 100 min) Data showing log amplification plot across a set of nine 2-fold serial dilutions of hgDNA ranging from 10 ng to 0.039 ng per reaction, using a standard 2-step cycling protocol (10 min at 95 ºC activation, 40 cycles of 15 sec at 95 ºC and 60 sec at 60 ºC, 60 ºC – 95 ºC melt). 20 μL reactions were performed using KAPA SYBR® FAST qPCR Master Mix on the Eppendorf realplex4 S realtime PCR system. Data represents 5 replicates for each DNA dilution. The ss48422848 assay targets a 150 bp amplicon.
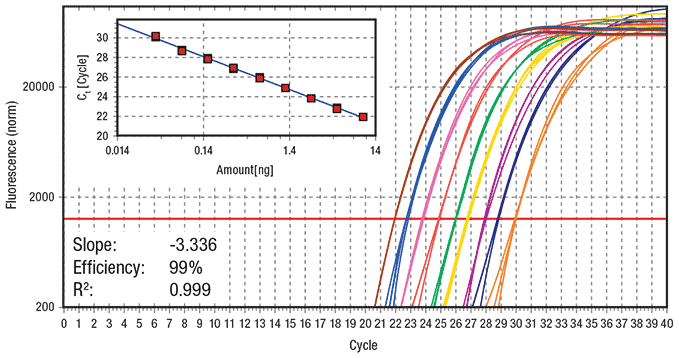
Figure 3.Fast cycling protocol (Run time = 44 min) Data showing log amplification plot across a set of nine 2-fold serial dilutions of hgDNA ranging from 10 ng to 0.039 ng per reaction using a fast 2-step cycling protocol (3 min at 95 ºC activation, 40 cycles of 3 sec at 95 ºC and 20 sec at 60 ºC, 60 ºC – 95 ºC melt). 20 μL reactions were performed using KAPA SYBR® FAST qPCR Master Mix on the Eppendorf realplex4 S real-time PCR system. Data represents 5 replicates for each DNA dilution. The ss48422848 assay targets a 150 bp amplicon.
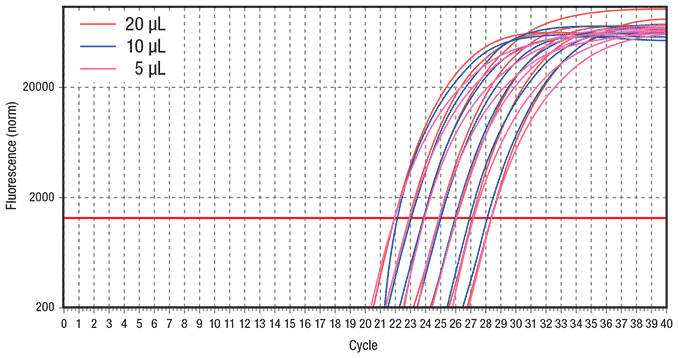
Figure 4.Comparison of different qPCR reaction volumes Similar results were obtained when either 20 μL, 10 μL or 5 μL reactions were performed across a set of seven 2-fold serial dilutions of hgDNA ranging from 10 ng to 156.25 pg per reaction using a fast 2-step cycling protocol. Reactions were performed using KAPA SYBR® FAST qPCR Master Mix on the Eppendorf realplex4 S real-time PCR system. Data represents an average of duplicates for each DNA dilution. The ss48422848 assay targets a 150 bp amplicon.
Conclusion
High-performance, fast qPCR requires a real-time instrument capable of extremely fast ramp rates with excellent uniformity across all samples, and a qPCR reagent containing a DNA polymerase capable of synthesizing DNA significantly faster than wild-type Taq Polymerase. The combination of Eppendorf realplex4 S real-time PCR system and KAPA SYBR® FAST qPCR Kits provides an industry-leading solution for highperformance, fast qPCR without compromising reaction efficiency and reproducibility. In addition, the ability to perform low-volume qPCR can reduce running costs while maintaining high quality results.
Reference
Per continuare a leggere, autenticati o crea un account.
Non hai un Account?