P-Phos, PhanePhos and BoPhoz™ Ligands

The P-Phos ligand family was developed by Professor Chan of Hong Kong Polytechnic University and licensed to JM CCT in 2002. P-Phos is an atropisomeric biaryl bisphosphine with the unique feature of incorporating two methoxy-substituted pyridine rings in the backbone.1 This family of ligands often presents higher activity and selectivity than the analogous BINAP ligands in a series of reactions such as ruthenium catalyzed hydrogenation of β-ketoesters2 (Scheme 1) and rhodium- and ruthenium-catalyzed hydrogenation of dehydroamino acids (Scheme 2).3 Ru catalyzed hydrogenation of non-functionalized ketones is also highly effective using P-Phos ligands.4 Moreover, JM has further developed the scope of P-Phos Ru diamine complexes in ketone hydrogenation by the application of less traditional 1,3- and 1,4‑diamines (Scheme 3).5 Iridium-P-Phos catalysts have been recently used for the asymmetric hydrogenation of C=N bonds in quinolines (Scheme 4).6


Scheme 1.

Scheme 2.

Scheme 3.

Scheme 4.
Phanephos was first reported in 1997, and since then it has found applications in the rhodium-catalyzed hydrogenation of dehydroamino acids7 (Scheme 5) and the ruthenium-catalyzed hydrogenation of β-ketoesters (Scheme 6).8 Both rhodium and ruthenium catalysts bearing the Phanephos ligand show an exceptionally high activity in most homogeneous hydrogenation reactions.

Scheme 5.

Scheme 6.
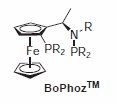
The BoPhoz class of ligands, based on the ferrocene backbone, have proven to be exceptionally active in many rhodium-catalyzed hydrogenations9 as well as being used in a number of rutheniumcatalyzed reactions. More recently Johnson-Matthey has developed new variants of BoPhoz ligands that offer increased structural and electronic diversity that may be required for the desired transformation. MeBoPhoz has shown excellent activity in many rhodium-catalyzed asymmetric hydrogenation of C=C bonds in dehydroaminoacids and α,β-unsaturated acids and esters (s/c up to 100,000) (Scheme 7).10

Scheme 7.
The P-Cy-Me-BoPhoz ligand has been shown to catalyze the asymmetric reduction of α-ketoesters, which is unusual for a rhodium catalyst (Scheme 8).

Scheme 8.
References
Per continuare a leggere, autenticati o crea un account.
Non hai un Account?