NHC-based Palladium Catalysts
In collaboration with Umicore AG and Co.,1 we are offering a series of robust Pd(II) and Pd(0) complexes used as the linchpin in C–C bond forming reactions. The high-performance Pd catalysts can efficiently couple alkyl- and aryl chlorides with organoboron compounds on large scale (100 g–100 t).2 The high TONs, mild reaction conditions, and economic viability/availability of aryl chlorides, make this methodology attractive to industrial scale applications. Catalysts 1 and 2 exhibit superior activity in C–C coupling reactions. They are formally Pd(0) and are rare examples of well-characterized monocarbene palladium precursors to 12-electron complexes. The Umicore NHC-Pd system performs Suzuki and Kumada couplings as well as α-arylation reactions at reasonable temperatures.
In the latter case [(NHC)Pd(allyl)Cl],2 a reactive, formally 16- electron complex, mediates the α-arylation of an array of aryl ketones (Scheme 1).3 The air-stable catalyst, short reaction times, and high conversions prove the usefulness of this NHC technology over previous Pd catalyzed α-arylations. The reactivity of both alkyl–alkyl and alkyl–aryl ketones was studied in this early NHC-Pd article from Nolan and co-workers.

Scheme 1. α-arylation of an array of aryl ketones
[{Pd(IMes)(NQ)}2] catalyst 2 demonstrated high reactivity and selectivity in sp3–sp2 Kumada couplings (Scheme 2).4 The generality of this methodology extends to both electron-rich and electron-poor arylmagnesium reagents. Furthermore, a broad spectrum of functionalized alkyl chlorides were used to afford complex organic building blocks. The high product yields at room temperature validates the robustness of the catalytic system versus well-known Pd-phosphine catalysts (Pd(PPh3)4, Pd2dba3) as a function of reaction conditions.

Scheme 2.Kumada couplings
The related [{Pd(IPr)(NQ)}2] catalyst 1 exhibited impressive activity in the Suzuki-Miyaura coupling of aryl chlorides with phenyl boronic acids (Scheme 3). At 50 °C, the high-yielding (88%) reaction was complete in one hour at a catalyst loading of 0.5 mol %.5 Interestingly, Pd(0) catalyst 1 produced lower yields of coupled biaryl product at room temperature, whereas analogous catalyst 2 gave 86% yields of 4-Cl-biphenyl at both room temperature and 50 °C under identical conditions. Presumably, catalyst 1 needs additional energy to climb over the activation barrier and enter the catalytic cycle as a naked Pd-NHC species. It should be noted that the reactivity of [{Pd(IPr)(NQ)}2] was also shown to be high in the coupling of sterically encumbered 2,6-diphenyl chloride and 1-naphthaleneboronic acid.

Scheme 3. Suzuki-Miyaura coupling of aryl chlorides
Beller and co-workers established a reactivity profile for NHC-Pd naphthoquinone catalysts in Heck reactions (Table 1).6 The outstanding capacity of this system is illustrated in the following scheme, wherein the best stilbene yields were obtained at 140 °C in an ionic liquid medium. The low catalyst loading (0.5 mol %), cheap aryl chloride reagents, and a stabilized ionic liquid environment contribute to the potential advancement of this chemistry to the industrial fine chemicals arena.
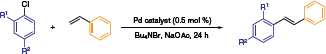
References
Per continuare a leggere, autenticati o crea un account.
Non hai un Account?