Blazar® Rapid Virus Detection
Our proprietary Blazar® platform is an advanced multiplex and degenerate PCR technology for adventitious and species-specific virus detection that is in line with current regulatory guidance. The Blazar® platform is a targeted molecular method that provides accurate and highly sensitive viral detection in days. By amplifying multiple targets within a conserved region of the viral family genome, our clients can detect a broader range of viruses compared to traditional PCR methods.
- ✓ FAST - Screen for viruses within days, and release batches sooner incorporating lab automation and digital reporting
- ✓ SENSITIVE - detect viruses, and any variants, down to as little as 10 genomic copies
- ✓ SAFE - fully validated and GMP compliant procedure
Related Product Literature
- Alternatives to in vivo assays for biosafety testing of biologics
Here we examine the current in vivo methods and explore alternatives which can be employed today. We also propose that while the industry may be some years away from removing in vivo testing completely, a case can be made for removing animal use from well-characterized production systems such as CHO.
- Accelerating Bulk Harvest Testing using the Blazar® CHO Animal Origin Free Virus Panel for Rapid Adventitious Virus Detection
This white paper describes the Blazar® CHO Animal Origin Free (AOF) panel for rapid adventitious virus detection, a targeted molecular method for broad virus screening.
Applications of the Blazar® platform
| Blazar® Platform | Description | Related Services | Modality | Compliance level | Turnaround Time |
|---|---|---|---|---|---|
| Current State | |||||
| Blazar® Rodent panel | A sustainable alternative to traditional in vivo MAP/HAP/RAP test. Reducing the use of animal models. | Cell Banking, Cell Line Characterization, and Biorepository | Biologics (monoclonal antibodies and recombinant proteins) | GMP | Conventional >35 days, now 14 days |
| Blazar® CHO AOF panel | Designed as a suitable replacement for the in vitro assay for CHO bulk harvest testing, providing rapid results with appropriate breadth of detection (plus variants) and sensitivity | Bulk Harvest Release Testing | Biologics (monoclonal antibodies and recombinant proteins) | GMP | Conventional 35 days, now 12 days |
| Future State | |||||
| Blazar® panel with Targeted NGS | Combines the breadth of detection of targeted next generation sequencing (NGS) with the speed and sensitivity of PCR methods | Cell Banking, Cell Line Characterization, and Biorepository Bulk Harvest Release Testing Drug Substance, Drug Product Release Testing, and Stability Testing | Biologics (monoclonal antibodies and recombinant proteins) Cell & Gene Therapies | GMP |
A multiplex approach that goes beyond single target virus detection
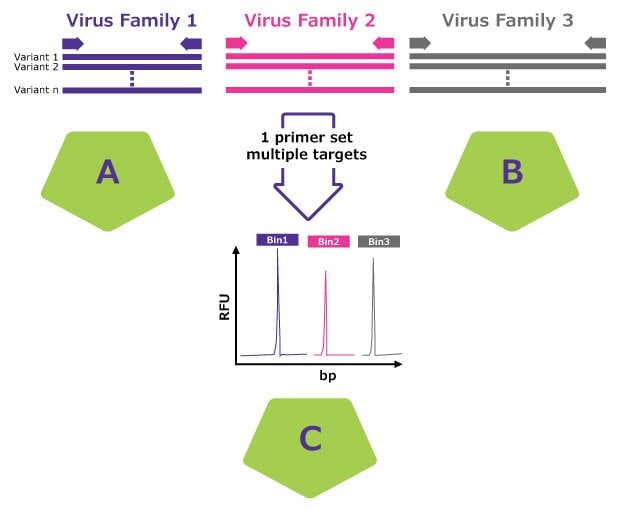
A: Degenerate primers broaden specificity
B: Nested PCR approach enhances sensitivity
C: Fragment detection by capillary electrophoresis. Internal positive controls spiked into each test sample
The Blazar® platform will enable next generation bioprocessing strategies – from process intensification, to connected processing, to fully continuous manufacturing – expediting biomanufacturing timelines thorough enhanced productivity.
Maintain a competitive advantage by accelerating your biologics program with the Blazar® platform for rapid virus detection.
Use the form below to learn more about maintaining a competitive advantage and accelerating your biologics program with the Blazar® platform.
To continue reading please sign in or create an account.
Don't Have An Account?