SAB4200711
Anti-Human Serum Albumin antibody, Mouse monoclonal
clone HSA-11, purified from hybridoma cell culture
Synonym(s):
ALB
About This Item
Recommended Products
biological source
mouse
antibody form
purified from hybridoma cell culture
antibody product type
primary antibodies
clone
HSA-11, monoclonal
form
buffered aqueous solution
mol wt
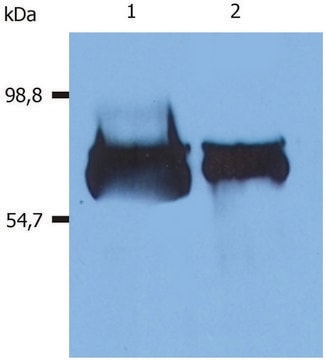
~70 kDa
species reactivity
gibbon, monkey, baboon
concentration
~1.0 mg/mL
technique(s)
immunoblotting: 2.5-5 ng/mL using human serum
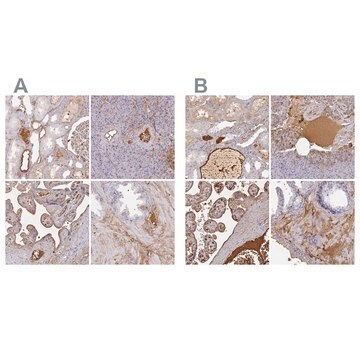
immunohistochemistry: suitable
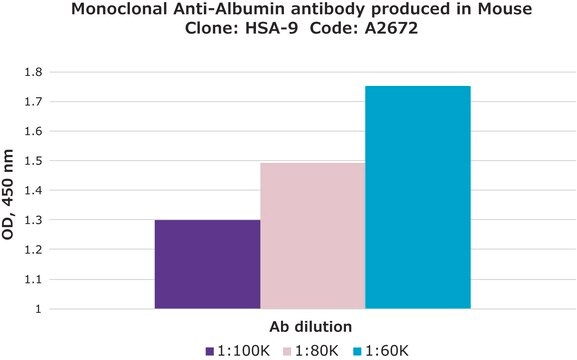
indirect ELISA: 0.2-0.4 μg/mL using 10 μg/ml Human Serum Albumin for coating
isotype
IgG2a
UniProt accession no.
shipped in
dry ice
storage temp.
−20°C
target post-translational modification
unmodified
Gene Information
human ... ALB(213)
Related Categories
General description
HSA is encoded by the gene mapped to human chromosome 4q13.3. It is characterized with three homologous domains that assemble to form a heart-shaped molecule. HSA constitutes around 60-65% of total serum protein.
The human serum albumin (HSA) gene is located on human chromosome 4. It encodes a globular protein that is the most prominent protein in human sera. It reaches a total concentration of about 60% of the total proteins in the blood serum. The globular protein is composed of 585 amino acids and comprises of three globular domains that resemble each other in structure, each containing two subdomains.
Specificity
Immunogen
Application
Biochem/physiol Actions
Human serum albumin (HSA) is a single non-glycosylated chain that has excellent binding capacity for various endogenous and exogenous ligands. It functions as a plasma transporter molecule and primarily binds to non-esterified long-chain fatty acids. It also binds to and transports various metabolites, such as bilirubin, steroid hormones, thyroxine, tryptophan, certain vitamins and metal ions within the body. It also has the ability to bind to several drugs and affects their pharmacokinetics and pharmacodynamics. HSA functions as a NO-carrier and is also responsible for the antioxidant capacity of human serum. In cases of acute hemolysis, HSA binds to heme in the blood stream and transports to hemopexin, where in it is reabsorbed by parenchymal liver cells.
Physical form
Storage and Stability
Disclaimer
Not finding the right product?
Try our Product Selector Tool.
Storage Class Code
10 - Combustible liquids
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service