All Photos(1)
About This Item
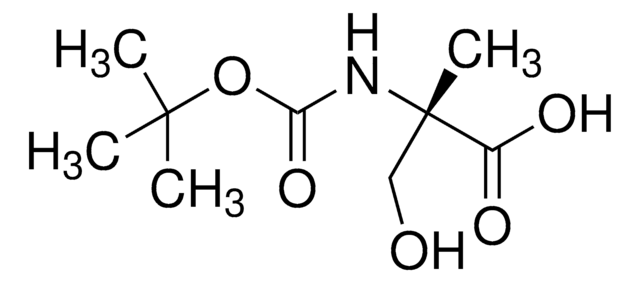
Empirical Formula (Hill Notation):
C4H9NO3
CAS Number:
Molecular Weight:
119.12
EC Number:
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Assay
≥98% (TLC)
form
powder
color
white
SMILES string
CC(N)(CO)C(O)=O
InChI
1S/C4H9NO3/c1-4(5,2-6)3(7)8/h6H,2,5H2,1H3,(H,7,8)
InChI key
CDUUKBXTEOFITR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Biochem/physiol Actions
α-Methyl-DL-serine is an amino acid derivative.
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
H Mickos et al.
Acta chemica Scandinavica (Copenhagen, Denmark : 1989), 46(10), 989-993 (1992-10-01)
The three-dimensional structure of the RGD-adhesion sequence has been studied previously by means of linear and cyclic peptides. These peptides show widely differing affinities to integrins, ascribed to a strong dependence on steric factors in the receptor recognition. Insertion of
Francisco Corzana et al.
Chemical communications (Cambridge, England), 47(18), 5319-5321 (2011-04-01)
A novel Tn antigen mimic, in which the natural underlying amino acid has been replaced by the non-natural α-methylserine analogue, is reported. This derivative exhibits a similar affinity for a natural lectin as for the natural Tn and retains the
Ewa Zabłotna et al.
Biochemical and biophysical research communications, 340(3), 823-828 (2005-12-29)
In many complexes formed by serine proteinases and their inhibitors, the hydroxyl group provided by water molecule or by the inhibitor Ser residue is located close to the inhibitor P1-P1' reactive site. In order to investigate the role of this
Yong-Chul Jeong et al.
Organic & biomolecular chemistry, 9(19), 6663-6669 (2011-08-17)
An efficient strategy for the control of the chemoselectivity in Dieckmann ring closures leading to tetramic acids derived from serine and α-methyl serine is reported, and this provides pathways to diversely substituted systems from a common starting material.
An approach to enantioselective activation of N-benzoyl-alpha-methylserine with chiral N-triazinylammonium chloride.
Beata Kolesińska et al.
Acta poloniae pharmaceutica, 63(5), 426-429 (2007-03-16)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service