L5647
Lidocaine hydrochloride monohydrate
≥99% (HPLC), solid, Na⁺ channel blocker
Synonym(s):
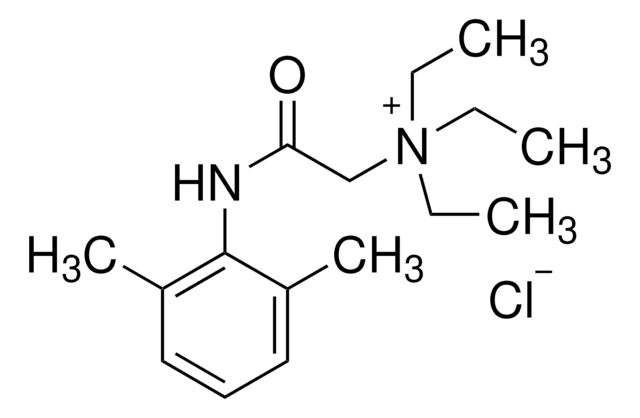
2-Diethylamino-N-(2,6-dimethylphenyl)acetamide hydrochloride monohydrate, Lignocaine hydrochloride monohydrate, Xylocaine hydrochloride monohydrate
About This Item
Recommended Products
product name
Lidocaine hydrochloride monohydrate, solid
form
solid
color
white
solubility
H2O: soluble
SMILES string
Cl[H].[H]O[H].CCN(CC)CC(=O)Nc1c(C)cccc1C
InChI
1S/C14H22N2O.ClH.H2O/c1-5-16(6-2)10-13(17)15-14-11(3)8-7-9-12(14)4;;/h7-9H,5-6,10H2,1-4H3,(H,15,17);1H;1H2
InChI key
YECIFGHRMFEPJK-UHFFFAOYSA-N
Gene Information
human ... SCN10A(6336) , SCN11A(11280) , SCN1A(6323) , SCN2A(6326) , SCN3A(6328) , SCN4A(6329) , SCN5A(6331) , SCN7A(6332) , SCN8A(6334) , SCN9A(6335)
Looking for similar products? Visit Product Comparison Guide
General description
Application
- in the preparation of drug composite for the controlled delivery in vitro, evaluated by drug release profile in ultra-high performance liquid chromatography (UPLC) and as a reference standard for fourier transform infrared spectroscopy (FT-IR).
- to record dose dependent response of sodium voltage (Nav1.7) channels in the injection-molded polymer device.
- For inhibition of glial cells in brain tissue samples.
Biochem/physiol Actions
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service