C8890
4-Chloro-1-naphthol
horseradish peroxidase substrate, fluorogenic, ≥98% (GC), crystalline
Synonym(s):
4-chloronaphthalen-1-ol
About This Item
Recommended Products
product name
4-Chloro-1-naphthol, crystalline
Assay
≥98% (GC)
form
crystalline
mp
118-121 °C (lit.)
solubility
acetone: 50 mg/mL, clear, colorless to very faintly brown
storage temp.
−20°C
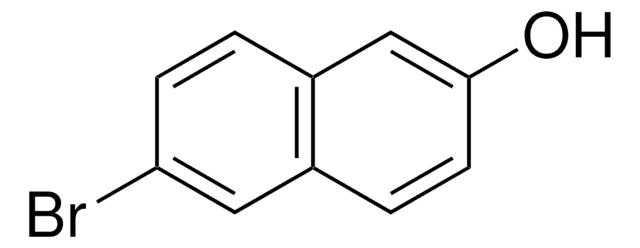
SMILES string
Oc1ccc(Cl)c2ccccc12
InChI
1S/C10H7ClO/c11-9-5-6-10(12)8-4-2-1-3-7(8)9/h1-6,12H
InChI key
LVSPDZAGCBEQAV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Biochem/physiol Actions
Substrates
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
NBT-BCIP substrate system aids in western blotting and immunohistological staining, producing a blue-purple insoluble end product.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service