Protocol for Imaging of ATP-LW Staining
Preparing ATP-LW dye stock solution
1. Dissolve the contents of one vial (1 mg) of ATP-LW (Molecular Weight: 833.82) in 120 µl of DMSO to create a 10mM solution. Store the stock solution at ≤–20°C for longer periods.
Labeling cells
1. Culture cells in an appropriate medium and vessel for fluorescence microscopy.
2. Dilute the ATP-LW stock solution 1:1,000 ~ 1:50,000 in culture medium to prepare the ATP-LW staining solution.
3. Remove the medium.
4. Add sufficient staining solution to cover the cells.
5. Incubate for 30 minutes, protected from light. Change to culture medium.
6. Image the cells.
Spectral information and storage for ATP-LW
For ATP detection: Excitation/Emission (nm) 450nm/550nm
For Peroxynitrite detection: Excitation/Emission (nm) 520nm/586nm
Storage conditions: 2–8°C or ≤–20°C
Summary of key steps and results:
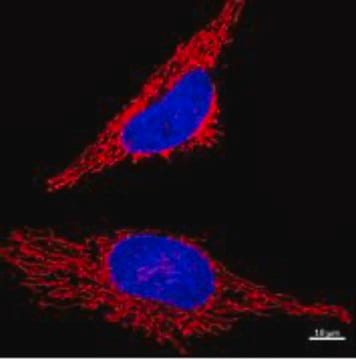
- HeLa cells were incubated with Camptothecin for 2h, then ATP-LW probe (30μM) for 30 min.
- ATP-LW probe works at 15µM~30µM for 30min staining on live Hela cells.
- Cells preincubated with ATP-LW probe (30μM) exhibited bright fluorescence after treatment with the ONOO− generator 3-morpholinosydnonimine hydrochloride (SIN-1).
- Green fluorescence increased after treatment with Camptothecin in Hela Cells.