557781
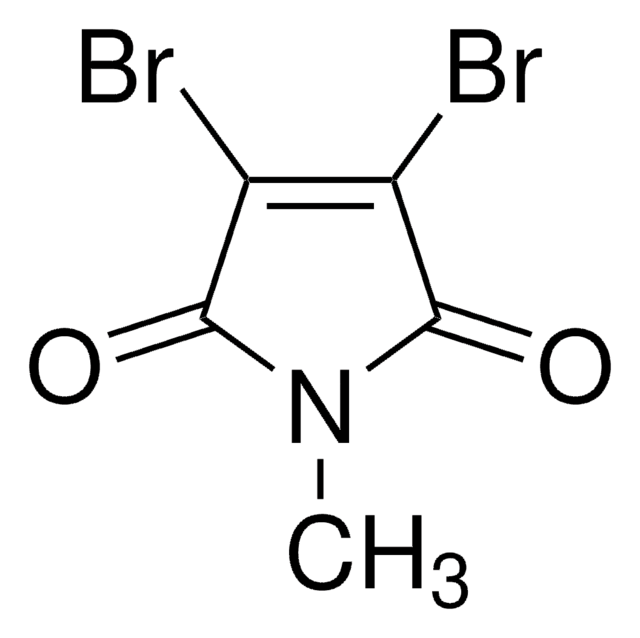
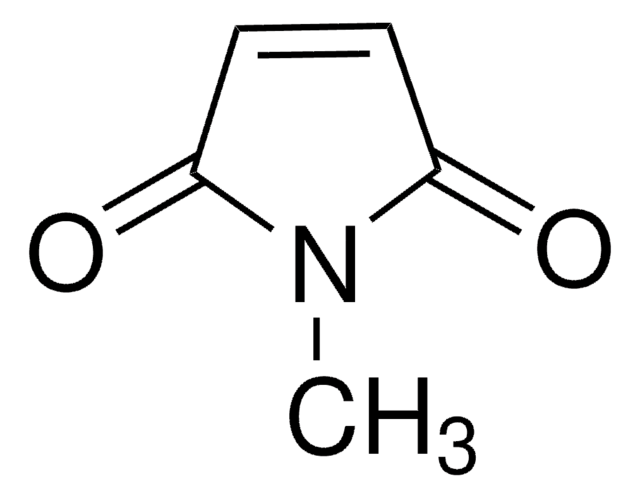
N-Benzyl-2,3-dibromomaleimide
97%
Synonym(s):
1-Benzyl-3,4-dibromo-pyrrole-2,5-dione
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C11H7Br2NO2
CAS Number:
Molecular Weight:
344.99
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
117-120 °C (lit.)
functional group
bromo
imide
maleimide
phenyl
SMILES string
BrC1=C(Br)C(=O)N(Cc2ccccc2)C1=O
InChI
1S/C11H7Br2NO2/c12-8-9(13)11(16)14(10(8)15)6-7-4-2-1-3-5-7/h1-5H,6H2
InChI key
DZYLZHRCCNTQCS-UHFFFAOYSA-N
Application
N-Benzyl-2,3-dibromomaleimide may be used in the synthesis of the following maleimide-based dyes, which can be employed in fluorescence quenching:
- 2,3-bis(3-indolyl)-N-benzylmaleimide
- 2,3-bis(2′-methyl-3-indolyl)-N-benzylmaleimide
- 2,3-bis(2-pyrrolyl)-N-benzylmaleimide
- 2-aryl-N-benzyl-3-bromomaleimides via suzuki cross coupling reaction with aryl boronic acid
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B - Skin Sens. 1
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
"Metal-catalyzed cross-coupling reactions of halomaleic anhydrides and halomaleimides: synthesis of structurally interesting and biologically important natural and unnatural products"
Deore SP and Argade PN
Synthesis, 46(03), 281-289 (2014)
Bifunctional maleimide dyes as selective anion sensors.
Lin Z, et al.
Tetrahedron, 65(27), 5216-5221 (2009)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service