All Photos(1)
About This Item
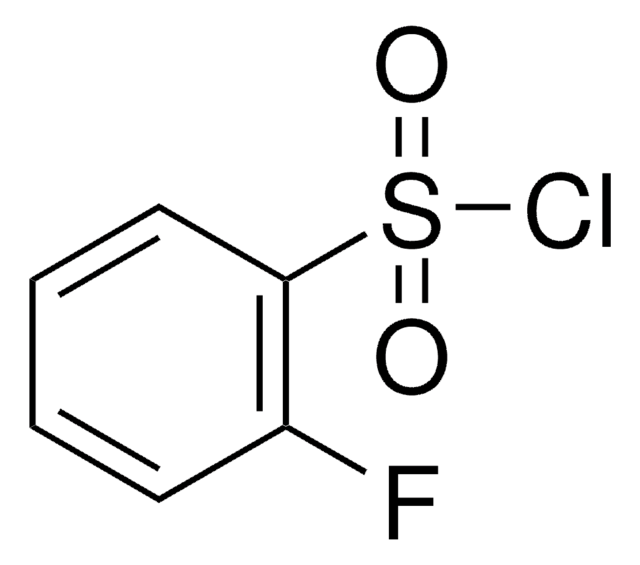
Linear Formula:
F2C6H3SO2Cl
CAS Number:
Molecular Weight:
212.60
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
refractive index
n20/D 1.518 (lit.)
bp
236-237 °C (lit.)
density
1.581 g/mL at 25 °C (lit.)
SMILES string
Fc1ccc(c(F)c1)S(Cl)(=O)=O
InChI
1S/C6H3ClF2O2S/c7-12(10,11)6-2-1-4(8)3-5(6)9/h1-3H
InChI key
FJSAJUXIHJIAMD-UHFFFAOYSA-N
General description
2,4-Difluorobenzenesulfonyl chloride is an aryl sulfonyl chloride derivative.
Application
2,4-Difluorobenzenesulfonyl chloride may be used in the preparation of 2,4-difluoro-N,N-dimethylbenzenesulfonamide, a precursor for poly(aryl ether sulfonamide)s.
It may be used in the preparation of the following benzenesulfonamide quinoline derivatives with potent anti-HIV-1 (human immunodeficiency virus type 1) activity:
It may be used in the preparation of the following benzenesulfonamide quinoline derivatives with potent anti-HIV-1 (human immunodeficiency virus type 1) activity:
- 2,4-difluoro-N-methyl-N-(quinolin-8-yl)benzenesulfonamide
- N-ethyl-2,4-difluoro-N-(quinolin-8-yl)benzenesulfonamide
- 2,4-difluoro-N-(quinolin-8-yl)benzenesulfonamide
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Fudi Zhong et al.
Organic & biomolecular chemistry, 13(6), 1792-1799 (2014-12-17)
Human immunodeficiency virus type 1 (HIV-1) Rev protein facilitates the export of viral RNA from nucleus to cytoplasm, which is a key step in HIV-1 pathogenesis and transmission. In this study, we have screened a commercial library and identified the
Sulfonamide as an Activating Group for the Synthesis of Poly (aryl ether sulfonamide) s by Nucleophilic Aromatic Substitution.
Rebeck NT and Knauss DM.
Macromolecules, 44(17), 6717-6723 (2011)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service